- What Causes Chickenpox and Herpes
- Key Differences at a Glance
- Symptoms Comparison
- Latency and Recurrence
- Transmission Pathways and Silent Spread
- Diagnosis and Laboratory Testing
- Complications and Long-Term Effects
- Transmission Routes and Infectious Stages
- Cure -Ayurvedic Antiviral
- Long-Term Complications if Left Untreated
- Frequently Asked Questions
- Reference
Chickenpox and herpes are caused by different viruses from the same herpes family. Chickenpox spreads through the air and usually occurs once in childhood, while herpes spreads through skin contact and causes recurrent outbreaks due to nerve latency.
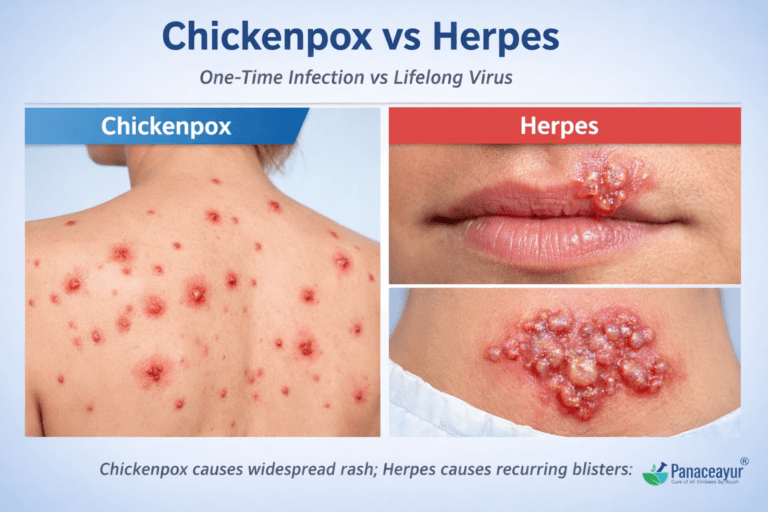
Chickenpox and herpes are often confused because both cause blister-like rashes and belong to the herpes virus family. However, they are not the same disease, do not spread the same way, and require completely different treatment approaches.
Chickenpox is usually a one-time childhood infection caused by the varicella-zoster virus, while herpes is a lifelong recurring infection caused by herpes simplex virus type 1 or 2. Misunderstanding this difference leads to delayed diagnosis, wrong treatment, and unnecessary fear.
This article clearly explains the difference between chickenpox and herpes, compares symptoms, transmission, recurrence, testing, and long-term risks, and also explains how Ayurveda approaches viral eradication instead of suppression, especially in chronic herpes cases.
Chickenpox vs herpes is a common confusion. This guide explains key differences, symptoms, diagnosis, and Ayurvedic treatments for lasting viral recovery. Chickenpox and herpes are often confused due to similar symptoms like skin rashes and fluid-filled blisters. However, they are caused by entirely different viruses—chickenpox by varicella-zoster virus (VZV) and herpes by herpes simplex virus (HSV-1 and HSV-2). While chickenpox typically occurs once in childhood, herpes is a chronic condition that can reactivate for years. Proper diagnosis is essential to avoid confusion and mistreatment. This article will walk you through the symptoms, diagnostic methods, long-term risks, and Ayurvedic Cure that address both conditions at the root level.
Chickenpox is caused by the Varicella-Zoster Virus (VZV), which belongs to the Herpesviridae family. Once a person recovers, the virus becomes dormant in nerve tissue and may later reactivate as shingles (herpes zoster), especially in older adults or immunocompromised individuals [1]. On the other hand, herpes is most commonly caused by Herpes Simplex Virus types 1 and 2 (HSV-1 and HSV-2). These viruses also lie dormant in the nervous system and are prone to frequent reactivation, particularly under stress, fever, or immune suppression [2].
While chickenpox is typically a self-limiting childhood illness, herpes represents a lifelong and often recurring condition that can have emotional, sexual, and reproductive implications [3]. Adding to the confusion is the fact that both viruses share the same viral family and the term “herpes” is often used loosely, leading many to mistakenly believe that chickenpox is a form of genital herpes or vice versa [4].
From an Ayurvedic perspective, these two conditions are also viewed very differently. Chickenpox aligns with Masurika or Laghu Masurika, a condition described in texts like Kashyapa Samhita and Bhavaprakasha, where the body manifests eruptive lesions due to deranged Pitta and Rakta dosha [5]. In contrast, herpes is categorized under Visarpa or Upadansha depending on its presentation, and is treated with antiviral herbs, Rasayana therapy, and Dhatu-level detoxification strategies [6].
This article explores the key differences between chickenpox and herpes from both modern virology and classical Ayurvedic standpoints. We will also examine symptoms, diagnosis, treatment options, co-infection risks (like CMV/EBV), and why proper understanding is critical—especially in a world where herpes is becoming increasingly common and misdiagnosed [7].
Chickenpox vs Herpes: Quick Comparison for Patients
Chickenpox is caused by the varicella-zoster virus and usually occurs once in life, mostly during childhood. It spreads through air droplets and causes a widespread itchy rash.
Herpes is caused by herpes simplex virus type 1 or 2. It is a lifelong infection, spreads through direct skin or sexual contact, and causes recurrent painful blisters, often without visible symptoms.
If your rashes keep coming back, involve nerve pain, or affect the mouth or genitals, it is not chickenpox, it is most likely herpes.
CHICKENPOX VS HERPES – QUICK COMPARISON
| Feature | Chickenpox | Herpes |
|---|---|---|
| Virus | Varicella-Zoster Virus (VZV) | HSV-1 / HSV-2 |
| First infection | Usually in childhood | Any age |
| Spread | Airborne | Skin & mucosal contact |
| Rash pattern | Whole body | Localized clusters |
| Recurrence | Shingles (later in life) | Recurrent outbreaks |
| Latency site | Dorsal root ganglia | Sensory nerve ganglia |
| Duration | 7–14 days | 5–10 days per episode |
| Nerve pain | Usually absent during primary infection | Common and appears before the lesions (burning, tingling, shooting pain) |
What Causes Chickenpox and Herpes
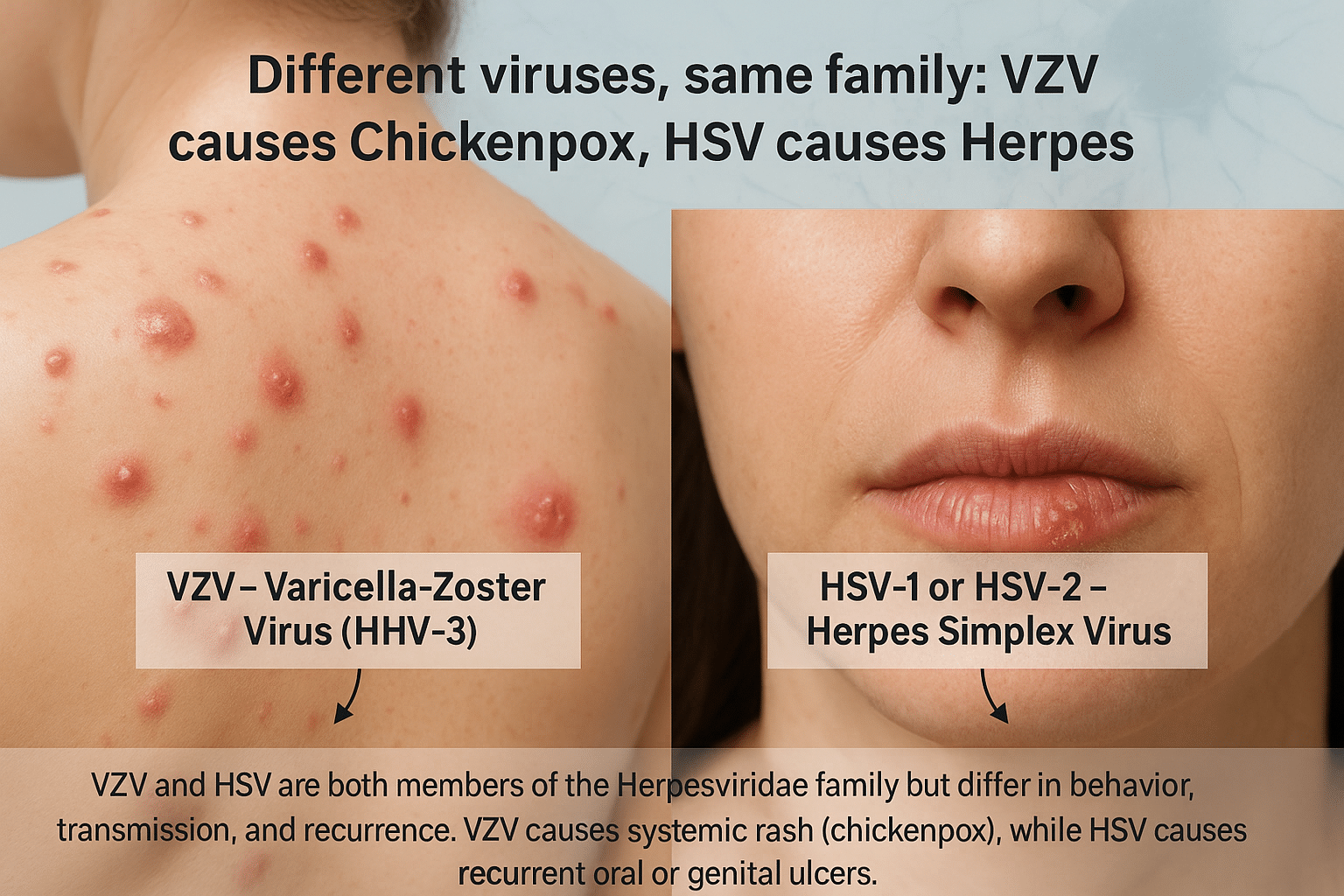
Although both chickenpox and herpes are caused by viruses from the same Herpesviridae family, they are distinct in origin, clinical course, and recurrence potential. Chickenpox is caused by the Varicella-Zoster Virus (VZV), also known as Human Herpesvirus 3 (HHV-3). It primarily affects children, presenting as an itchy, vesicular rash accompanied by fever and body aches. Once resolved, VZV remains dormant in the dorsal root ganglia and may reactivate years later as shingles (herpes zoster), especially in older adults or individuals with weakened immunity [8].
In contrast, herpes simplex refers to infections caused by Herpes Simplex Virus type 1 (HSV-1) and type 2 (HSV-2). HSV-1 typically manifests as cold sores around the lips and mouth, while HSV-2 is more associated with genital herpes. However, this distinction has blurred in recent years as HSV-1 is now a leading cause of genital herpes through oral-genital contact [9]. After the initial infection, HSV hides within the sensory nerve ganglia (trigeminal for HSV-1 and sacral for HSV-2) and can reactivate multiple times throughout life, often triggered by stress, illness, or menstrual cycles [10].
Both viruses share the ability to enter latency and evade the immune system, but their patterns of transmission, recurrence, and severity differ significantly. VZV typically infects a person once in life, followed by possible shingles decades later, while HSV establishes a lifelong cycle of periodic reactivation and viral shedding, often without symptoms [11]. From an Ayurvedic lens, chickenpox aligns with Masurika, while herpes is classified under Visarpa or Upadansha, depending on its clinical features, and requires a fundamentally different treatment path focusing on long-term viral latency and Ojas depletion [12].
Key Differences at a Glance
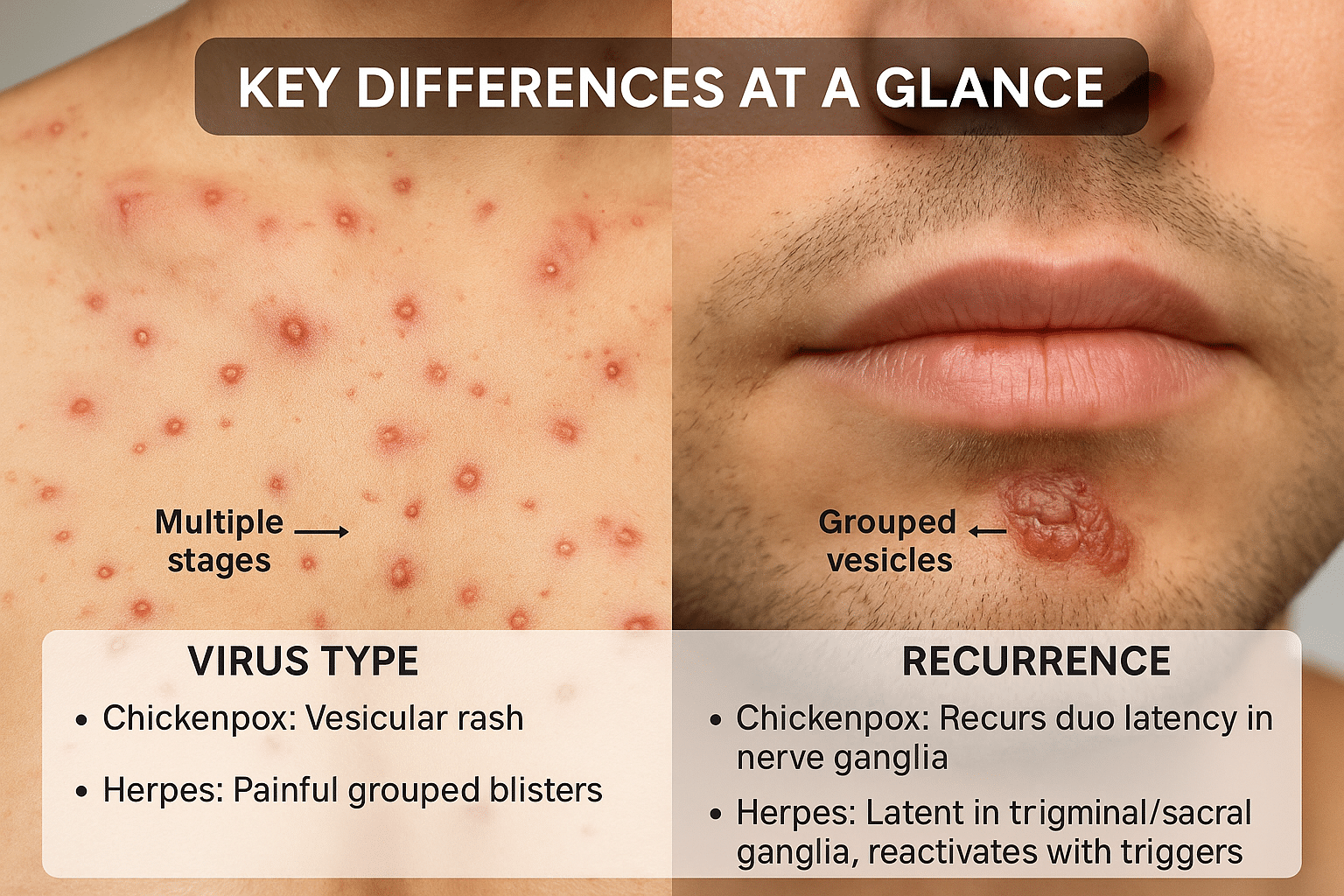
While both chickenpox and herpes originate from viruses in the Herpesviridae family, their behavior, symptoms, transmission, and treatment protocols vary significantly. One of the most noticeable differences is in the type of virus responsible: chickenpox is caused by Varicella-Zoster Virus (VZV or HHV-3), while herpes is caused by either HSV-1 or HSV-2, known respectively as Human Herpesvirus types 1 and 2 [13].
In terms of clinical presentation, chickenpox typically appears as a widespread, itchy rash that progresses through distinct stages—red macules, fluid-filled vesicles, pustules, and eventually scabs. These lesions usually cover the trunk, face, and limbs. In contrast, herpes presents as painful, grouped vesicles often localized to a particular area, such as the lips (oral herpes) or genitals (genital herpes), and does not follow the same progressive pattern [14].
The mode of transmission also differs. Chickenpox spreads through airborne respiratory droplets and direct contact with fluid from lesions, often making it highly contagious even before the rash appears. Herpes, on the other hand, is transmitted primarily through direct skin-to-skin or mucosal contact, such as kissing or sexual activity. Uniquely, herpes can be transmitted even in the absence of visible symptoms, due to asymptomatic viral shedding—a trait not observed with chickenpox [15].
Recurrence patterns sharply distinguish the two. Chickenpox generally occurs only once in a lifetime, with reactivation years later as shingles in some individuals. Herpes, however, is a lifelong condition with recurrent flare-ups, especially under conditions of physical stress, fever, or immunosuppression [16]. This makes herpes management significantly more complex, as it often requires long-term immune support.
From a treatment and prevention standpoint, vaccines are available for chickenpox (Varivax) and shingles (Shingrix), which significantly reduce incidence and severity. Currently, there is no approved vaccine for HSV, although experimental trials are underway. Ayurveda offers a personalized Rasayana-based protocol for both conditions, but the herpes protocol is more intensive and longer due to the tendency for reactivation and systemic immune disruption [17].
This foundational understanding of their key differences helps patients and practitioners approach both conditions more accurately—clinically, virologically, and therapeutically.
Symptoms Comparison
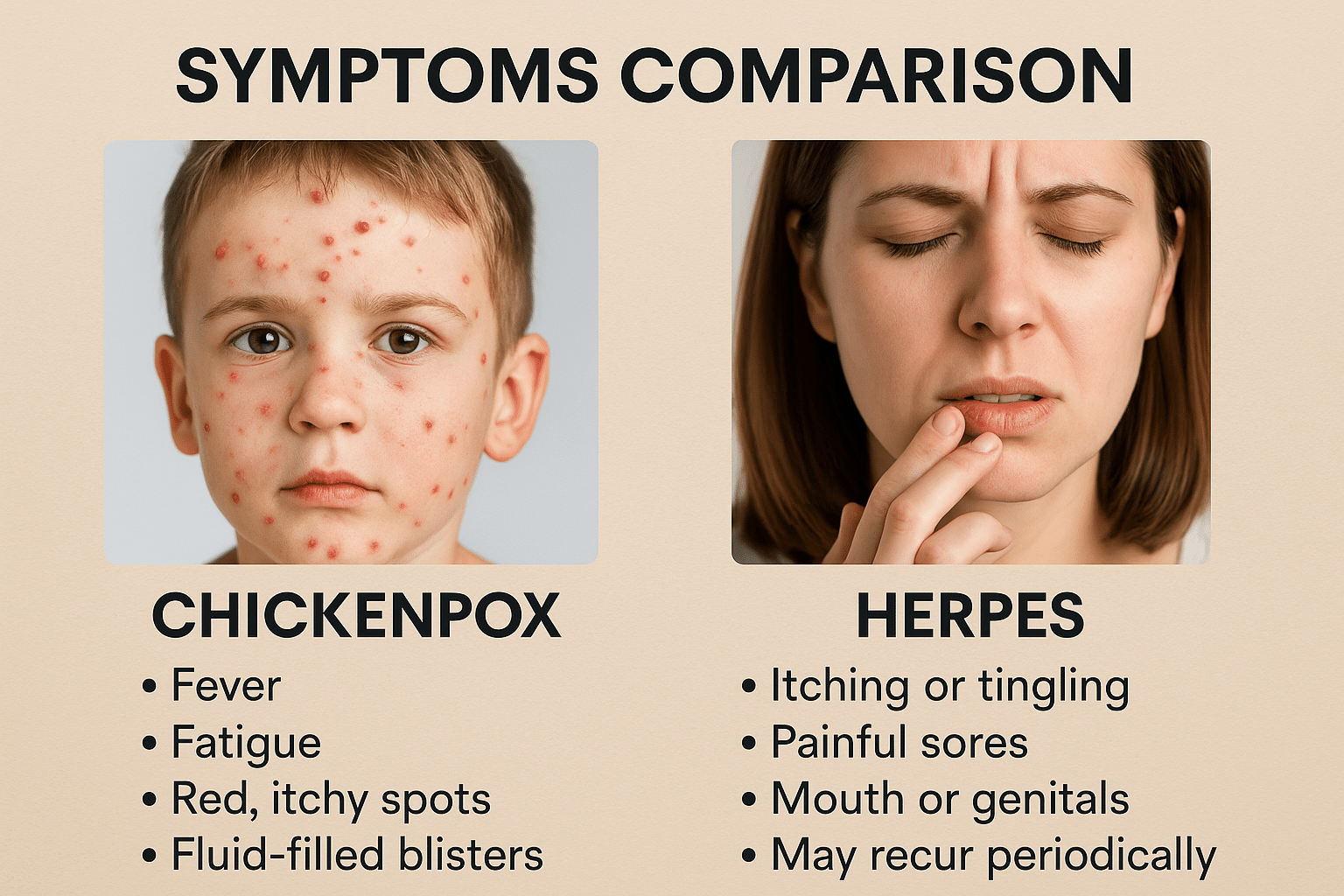
The symptom profile of chickenpox and herpes differs significantly in onset, distribution, and progression. Chickenpox typically begins with low-grade fever, body ache, fatigue, and irritability, followed by the rapid development of a widespread rash. The hallmark of chickenpox is that the lesions appear in multiple stages simultaneously including macules, papules, vesicles, and crusts often distributed over the face, trunk, and extremities. The rash is intensely itchy and may leave scars if scratched or secondarily infected. This multi-stage rash cycle is a clinical giveaway in differentiating it from herpes [18].
In contrast, herpes, whether oral (HSV-1) or genital (HSV-2)—typically starts with a burning, tingling, or itching sensation in the affected area, followed by the appearance of painful, clustered vesicles on an erythematous base. These vesicles may rupture into shallow ulcers before crusting over. Unlike chickenpox, herpes lesions tend to be localized, usually affecting the lips, genitalia, or nearby skin. The pain and tingling may be intense, especially during the first outbreak, and systemic symptoms like fever or swollen lymph nodes are more likely to occur during primary infections than recurrences [19].
An important clinical clue is the pattern of recurrence. While chickenpox usually occurs only once in a lifetime, herpes recurs due to viral latency in nerve ganglia. These recurrent outbreaks can be mild or severe and may happen every few weeks or months, particularly in immunocompromised individuals. Additionally, many herpes infections are asymptomatic, which allows silent transmission to others without the infected person even knowing they carry the virus [20].
From an Ayurvedic perspective, chickenpox (Masurika) is often associated with Pitta-Rakta aggravation manifesting as eruptive skin lesions, while herpes (Upadansha or Visarpa) is linked with Dushta Rakta, aggravated Pitta, and vitiated Kapha, causing burning, oozing, and relapsing vesicles [21]. Ayurvedic clinical features include daha (burning), kandu (itching), and sphutana (rupture of pustules), all pointing to different Dosha combinations in herpes and chickenpox. Consequently, the symptomatic approach and treatment principles are also distinct [22].
Latency and Recurrence
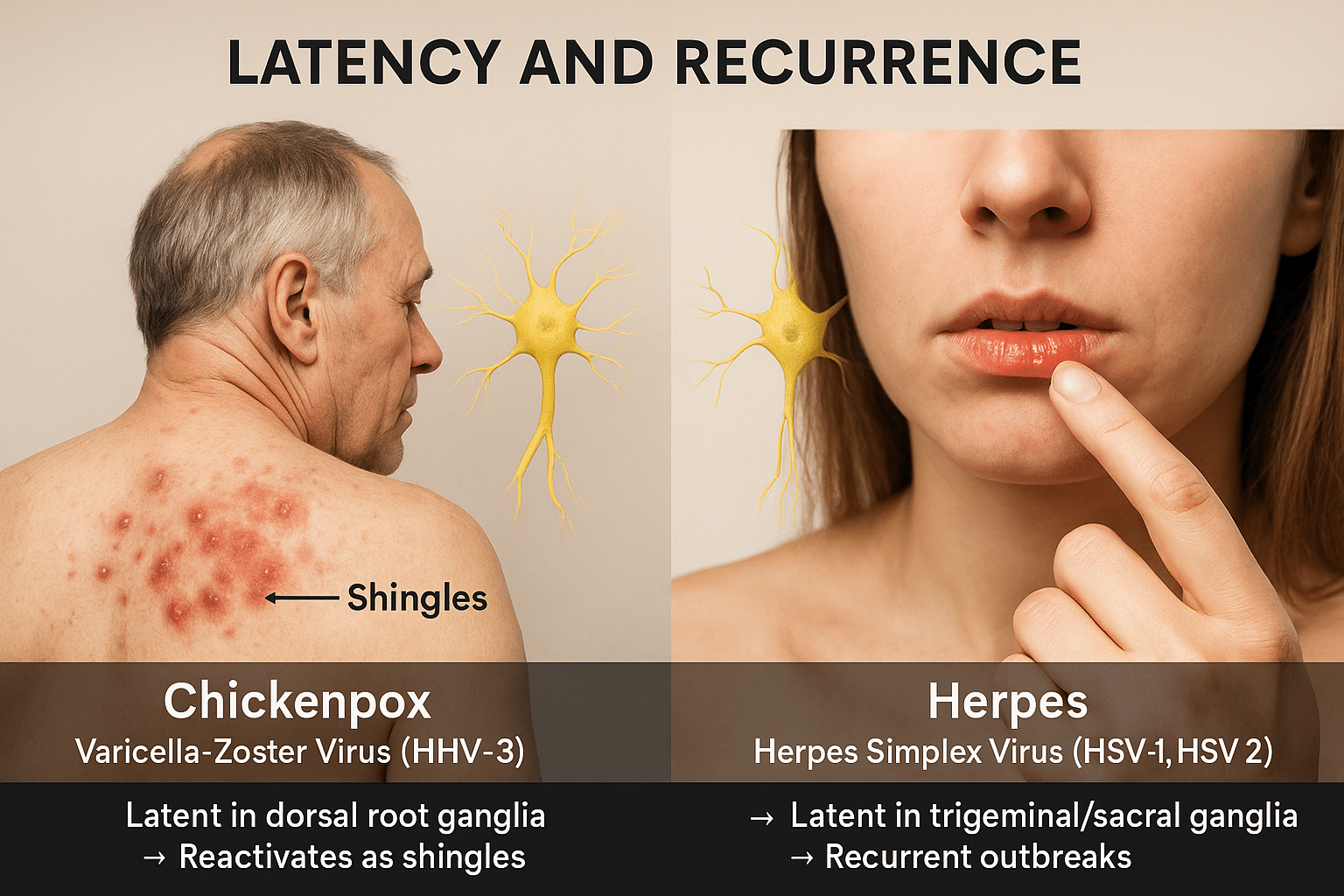
Latency in Chickenpox (VZV)
After the initial infection, Varicella-Zoster Virus (HHV-3) enters a dormant phase in the dorsal root or cranial nerve ganglia. In most individuals, this virus remains silent for life. However, under conditions such as aging, immunosuppression, or chronic stress, it may reactivate as herpes zoster (shingles). This reactivation typically appears once or twice in a lifetime and manifests as a painful, band-like rash limited to a single dermatome, reflecting the virus’s origin in the nervous system reservoirs [23].
Latency in Herpes (HSV-1 and HSV-2)
In contrast, Herpes Simplex Virus (HSV-1 and HSV-2) exhibits lifelong latency in nerve ganglia—with HSV-1 residing in the trigeminal ganglion and HSV-2 in the sacral ganglia. The virus can reactivate multiple times a year, often triggered by emotional stress, hormonal changes, fever, or UV exposure. Unlike chickenpox, herpes frequently returns with or without symptoms, and asymptomatic shedding is common, contributing to unnoticed transmission [24].
Ayurvedic View on Viral Latency
Ayurveda explains latency through the lens of Sookshma Avastha—a subtle stage where Dosha-dushya sammurchana (interaction of imbalanced Doshas and tissues) lodges the disease deep within Marmas and Srotas. For chickenpox (Masurika), the dormant phase is typically contained within the Rakta Dhatu, whereas for herpes (Upadansha or Visarpa), the latency persists in Rakta and Majja Dhatu, aggravated by Vata, Pitta, and Kapha imbalance [25].
Clinical Recurrence: Comparison
- Chickenpox (VZV): Single lifetime occurrence; reactivates rarely as shingles.
- Herpes (HSV-1/2): Frequent recurrences; triggered by minor stressors or immune changes.
This recurrence is not merely superficial. In Ayurveda, it signifies unresolved pathology at the Dhatu level, needing deeper interventions beyond surface symptom relief.
Treatment Implications in Ayurveda
Ayurvedic management of herpes latency includes:
- Rasayana herbs like Ashwagandha, Guduchi, and Bhumyamalaki
- Herbo-mineral formulations like Gandhak Rasayan, Heerak Bhasma, and Swarna Makshik
- Optional Shodhana therapies (e.g., Basti, Virechana) based on strength and patient Prakriti
- These aim not just to control symptoms but to eradicate the viral presence from its latent nerve reservoirs [26].
Modern research supports this view: HSV latency within neurons makes it largely inaccessible to common antivirals like acyclovir. Therefore, Ayurveda’s tissue-specific and Rasayana approach holds promise for deep eradication where conventional medicine cannot reach [27].
Transmission Pathways and Silent Spread
How Chickenpox Spreads
Chickenpox is primarily spread through respiratory droplets and direct contact with the vesicular fluid of lesions. The virus is highly contagious, especially during the early stage—1–2 days before the rash appears—and remains infectious until all blisters have crusted. It typically spreads rapidly in households and school environments, often affecting children who have not been vaccinated or previously exposed to the virus. The incubation period for chickenpox ranges from 10 to 21 days, and symptomatic individuals are typically advised to isolate until the contagious period ends [28].
How Herpes Spreads
Herpes Simplex Virus (HSV) spreads through skin-to-skin contact, including oral, genital, or anal contact, and can also be transmitted via saliva or mucosal membranes. HSV-1 is commonly spread through kissing or shared items like utensils and lip balms, especially during active cold sore episodes. HSV-2 is primarily spread through sexual contact. Unlike chickenpox, HSV can be transmitted even when no visible sores are present, due to asymptomatic viral shedding. This makes herpes much more elusive and persistent in the community, often transmitted unknowingly [29].
Silent Transmission and Recurrence
A major concern with herpes transmission is its asymptomatic nature in many carriers. Studies indicate that up to 80% of HSV-2 infections are unrecognized, meaning people may spread the virus without ever realizing they are infected. Additionally, HSV’s ability to recur silently, especially in genital tissues, contributes to ongoing transmission. Chickenpox, in contrast, is easier to track due to its distinct symptomatic phase and typically occurs only once in a lifetime [30].
Ayurvedic Insight on Transmission
Ayurveda views disease transmission through the lens of Sansargaja (contagious) and Upsargaja (environmental) factors. Chickenpox corresponds to Sansargaja Masurika, caused by exposure to infected individuals and imbalanced Pitta in the Rakta Dhatu. In contrast, herpes is often seen as Upadansha or Visarpa, with transmission due to contaminated sexual activity, doshic disturbance (mainly Pitta and Kapha), and weak Ojas.
Moreover, silent transmission in herpes is metaphorically aligned with Sookshma Roga Sthiti—the state where disease elements are hidden in the micro-channels and remain dormant until triggered. This Ayurvedic model helps explain why viral shedding can happen without visible lesions, requiring deep detoxification and Rasayana therapy to halt both recurrence and transmission [31].
Diagnosis and Laboratory Testing
Diagnosis of Chickenpox
The diagnosis of chickenpox is often clinical, based on the patient’s symptoms and characteristic skin presentation. The hallmark is the presence of itchy, fluid-filled blisters that appear in successive crops on the face, trunk, and limbs. These lesions go through all stages—macules, papules, vesicles, pustules, and crusts—simultaneously, which helps distinguish it from other rashes. A patient history of exposure and unvaccinated status further supports the diagnosis.
In uncertain cases, laboratory tests such as Tzanck smear, polymerase chain reaction (PCR) for VZV DNA, or direct fluorescent antibody (DFA) tests may be employed to confirm varicella-zoster virus infection. PCR testing remains the most sensitive and specific method to detect active or reactivated VZV infection, especially in immunocompromised individuals or adults presenting with shingles-like symptoms [32].
Diagnosis of Herpes Simplex Virus (HSV)
HSV diagnosis also begins with clinical observation—particularly the appearance of painful vesicular lesions on the lips (HSV-1) or genitals (HSV-2), often preceded by tingling, burning, or itching. However, due to asymptomatic cases and non-specific symptoms, laboratory confirmation is often necessary.
The most accurate test is HSV DNA PCR, typically performed on swab samples from active lesions or via blood. It has high sensitivity and can detect both HSV-1 and HSV-2 strains. Viral culture from the lesion fluid is another traditional method, though less sensitive. In cases where no visible sores are present, serologic tests (IgG and IgM antibodies) may help identify past or recent infections, though IgM is not always reliable and may result in false positives. PCR blood testing offers better insights for latent infections and recurrent viral activity [33].
Ayurvedic Diagnostics (Rogi–Roga Pariksha)
Ayurveda does not rely on modern labs but employs detailed diagnostic frameworks like:
- Ashtavidha Pariksha (Eightfold Examination): Pulse (Nadi), tongue (Jihva), stool (Mala), urine (Mutra), etc.
- Dashavidha Pariksha: Evaluation of Prakriti, Vikriti, Agni (digestive fire), Srotas, and Ojas.
Chickenpox (Masurika) is generally diagnosed by observing Pitta-Rakta symptoms such as high fever, vesicular rash, and inflammatory discharge. In contrast, herpes (Upadansha or Visarpa) is evaluated based on recurrent lesions, nerve irritation, chronic fatigue, and deep-seated Kapha-Pitta pathology affecting Majja Dhatu and Rasavaha Srotas.
Accordingly, Rasayana therapy and viral elimination strategies are personalized based on these evaluations—not just based on the site of the rash but on the Dhatu (tissue), Dosha, and Srotas involvement.
Complications and Long-Term Effects
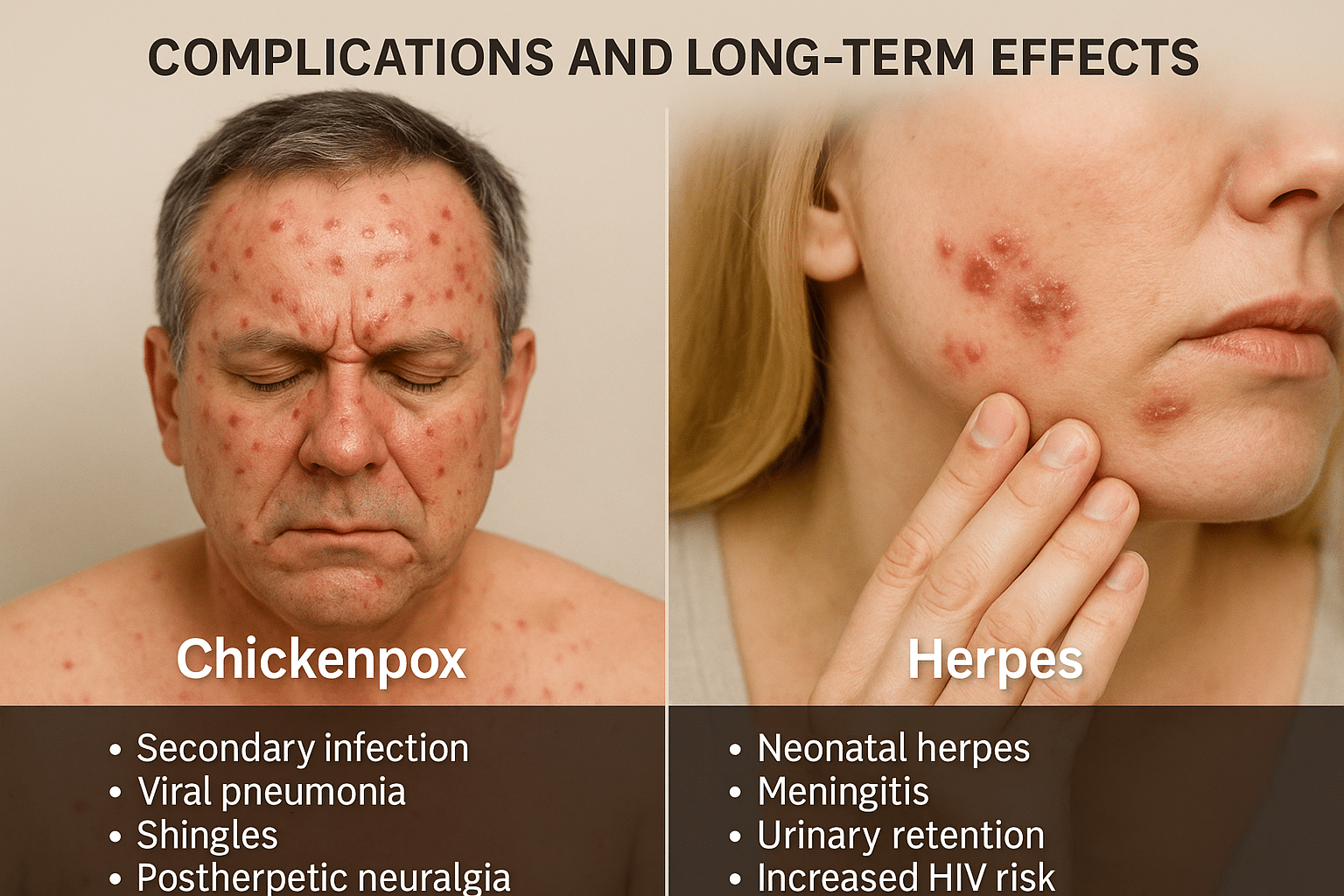
Chickenpox Complications
For most healthy children, chickenpox resolves on its own without major issues. However, in adults, infants, pregnant women, and immunocompromised individuals, the disease can lead to serious complications. These include secondary bacterial infections of the skin, viral pneumonia, hepatitis, and in rare cases, encephalitis (inflammation of the brain). A severe outcome of latent VZV reactivation is shingles, which can occur years after recovery. Shingles itself may lead to post-herpetic neuralgia (PHN)—a chronic, burning nerve pain that can persist for months or even years after the rash resolves, particularly in elderly individuals [34].
In pregnant women, VZV infection during the first or second trimester may result in congenital varicella syndrome, leading to birth defects, low birth weight, limb hypoplasia, or mental developmental delays in the fetus [35].
Herpes Complications
While herpes symptoms may seem mild in many patients, the long-term consequences can be severe, especially with frequent recurrences or untreated latent viral load. Genital herpes (HSV-2) is associated with an increased risk of HIV acquisition, chronic pelvic pain, urinary retention, and psychological distress. In newborns, transmission of HSV during childbirth can result in neonatal herpes, a potentially fatal condition that affects the central nervous system, liver, and lungs [36].
Over time, recurrent outbreaks can cause scarring, nerve hypersensitivity, and immune exhaustion, making the body more vulnerable to other viral infections like CMV, EBV, or HHV-6. Furthermore, asymptomatic shedding can result in unknowingly infecting sexual partners, contributing to the virus’s silent spread [37].
Ayurvedic Perspective on Long-Term Effects
In Ayurveda, long-standing herpes infections are seen as manifestations of Dushta Rakta (vitiated blood) and Majja Dhatu Kshaya (depletion of the nervous tissue). The repeated eruption of vesicles is a sign of Chronic Visarpa or Upadansha, where the body’s Agni (digestive and metabolic fire) is weakened, Ojas (vitality) is diminished, and Srotas (microchannels) are blocked due to unresolved viral load. If left untreated, this leads to deeper Dhatu-level damage, including nerve pain, sexual dysfunction, and weakened reproductive tissue [38].
In contrast, chickenpox complications like post-herpetic neuralgia are associated with Vata imbalance in the Majja and Rasa Dhatus, requiring pacification of Vata and nourishment through Basti, Rasayana therapy, and rejuvenating oils like Bala-Ashwagandha taila.
Ayurveda emphasizes preventive Rasayana for at-risk populations, including elderly, pregnant women, and immunocompromised individuals, to avoid both acute and chronic complications.
Transmission Routes and Infectious Stages
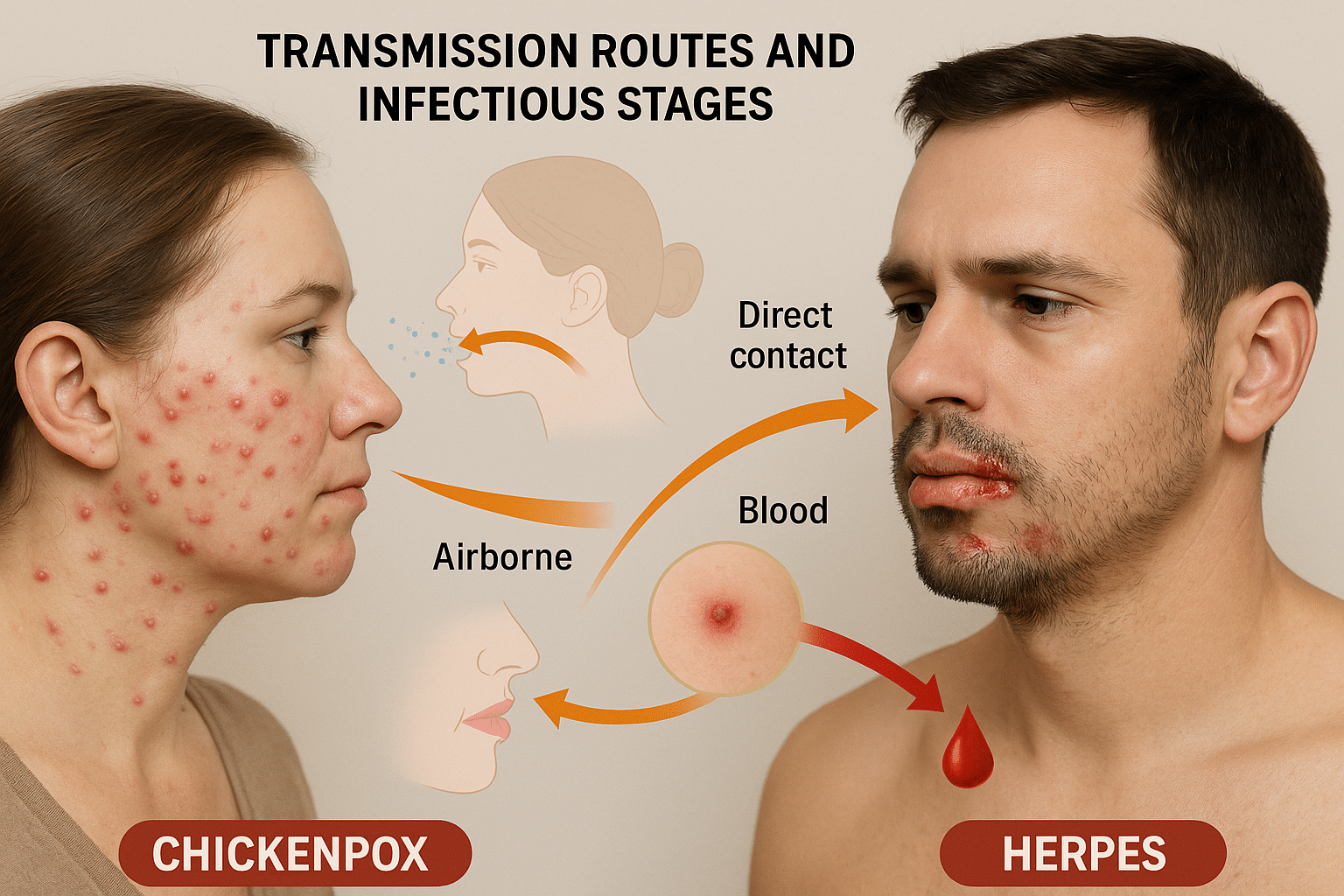
Chickenpox Transmission: Airborne and Contact-Based
Chickenpox, caused by the Varicella Zoster Virus (VZV), is among the most contagious viral infections known to medicine. The primary mode of transmission is via respiratory droplets expelled when an infected individual coughs, sneezes, or even talks. These droplets can remain suspended in the air for a prolonged period, especially in enclosed environments like schools, homes, and hospitals, posing a risk even without direct contact.
Additionally, direct contact with vesicle fluid from the skin lesions of an infected person can also cause transmission. This includes touching contaminated surfaces like bedsheets, towels, clothing, or toys—especially dangerous in pediatric settings.
The infectious window begins 48 hours before rash appearance and lasts until all the vesicles have crusted over, typically 5–7 days after onset. The incubation period ranges from 10 to 21 days, meaning the virus can silently spread within communities before being clinically detected [39].
Notably, individuals who recover from chickenpox remain lifelong carriers of dormant VZV within the sensory ganglia. This virus can reactivate years later, especially under stress or immunosuppression, as herpes zoster (shingles)—a painful and localized recurrence of the original infection [40].
Herpes Transmission: Skin Contact, Secretions, and Blood Entry
Unlike chickenpox, Herpes Simplex Virus (HSV-1 and HSV-2) is primarily spread through direct skin-to-skin or mucosal contact. This includes:
- Kissing (oral herpes/HSV-1)
- Oral-genital contact (cross-transmission of HSV-1 to genital region)
- Vaginal, anal, or genital intercourse (HSV-2 dominant)
- Touching infected lesions and then another person or body part
However, one of the most underappreciated routes of HSV transmission is through microscopic abrasions or mucosal breaches that allow direct access to the bloodstream. HSV virions can invade the bloodstream via these minor cuts or friction-induced micro-tears, which are often invisible to the naked eye, especially in high-friction mucosal areas like the genitals, anus, oral cavity, and cervix [41].
This makes blood transmission a significant yet overlooked mode, especially in cases of receptive intercourse, unnoticed menstrual abrasions, or shared sexual objects. Once inside the bloodstream, HSV disseminates via infected leukocytes and neurotropic invasion, seeking refuge in the dorsal root or trigeminal ganglia where it remains latent [42].
Asymptomatic viral shedding—a state where the virus is actively replicating and transmissible without outward signs—accounts for 70–80% of HSV transmission, making it a silent and persistent public health issue. Individuals may unknowingly infect partners, especially during early relationships or non-penetrative contact [43].
Clinical Context: Immune Evasion and Recurrence
Herpes is notorious for its immune evasion strategies. Once it infiltrates neural tissues, HSV integrates with host cells and downregulates MHC class I expression, avoiding immune detection. The virus periodically reactivates in response to triggers like fever, menstruation, sexual activity, emotional stress, lack of sleep, or immunosuppression, leading to symptomatic outbreaks or silent shedding.
This viral latency-reactivation cycle is absent in chickenpox (except during shingles), which makes HSV a chronic condition requiring long-term immune modulation, not just acute suppression.
Ayurvedic Interpretation of Viral Entry and Dormancy
In Ayurveda, chickenpox aligns with Masurika or Sansargaja Jwara, described in texts like Bhavaprakasha and Madhava Nidana. It is classified as a contagious febrile disorder (Sansargaja Vyadhi) involving Pitta and Rakta Dushti, often aggravated during Grishma and Varsha Ritu (summer and monsoon seasons). The route of transmission is believed to involve contact (Samsarga) and inhalation of disease-carrying air (Nishvasa Gata Visha), analogous to modern airborne droplet theory [44].
Herpes, on the other hand, is referenced under Upadansha, Visarpa, or Agantuka Kushtha, depending on the location and symptoms. The entry through contaminated blood or sexual fluid (Rakta–Shukra Dushti) aligns with the classical view that viruses can enter via broken skin, wounds, or weakened Dhatus (tissues). The virus resides in the Srotas (microchannels) and Majja Dhatu (nervous tissue), creating a Sookshma Rogasthiti (subclinical latency).
Ayurveda uniquely acknowledges the concept of Vyadhi Beeja (disease seeds) hiding in the body, akin to modern concepts of latent viral load. These dormant seeds are activated when Agni (metabolic fire) weakens, Ojas (immunity) depletes, or Manas (mental health) is disturbed. Therefore, management is not limited to suppression but involves strengthening host resistance through Rasayana, rejuvenation, and Virechana (detoxification) [45].
Ayurveda doesn’t treat viral infections like herpes or chickenpox with a single universal medicine. Instead, it addresses the root cause, dosha involvement, and tissue (dhatu) damage in each person. The herbs and minerals used in Ayurvedic medicine are prescribed based on your body constitution (Prakriti), immune strength, disease phase (acute or latent), and symptom profile. In chronic viral infections like HSV, CMV, or VZV, Ayurveda follows a unique three-step approach:
- Shodhana (cleansing): to expel deep-rooted doshas and toxins
- Shamana (palliative): to manage symptoms and balance the aggravated doshas
- Rasayana (rejuvenation): to repair immune fatigue and eliminate residual virus
Here’s a detailed look at the most powerful Ayurvedic herbs and minerals with antiviral action used in herpes and chickenpox care, and how they’re tailored per patient.
Key Ayurvedic Herbs with Proven Antiviral Effects
Guduchi (Tinospora cordifolia)
Guduchi is one of Ayurveda’s most revered Rasayanas, especially useful in managing latent viruses like HSV and CMV. It strengthens white blood cell activity, repairs DNA damage, and helps clear viral remnants from the blood (Rakta Dhatu) and nerves (Majja Dhatu) [46].
Bhumyamalaki (Phyllanthus niruri)
Widely used for viral hepatitis, Bhumyamalaki also shows potent antiviral effects against EBV and HSV. It detoxifies the liver, reduces systemic inflammation, and boosts antiviral enzymes. It is ideal for patients with a sluggish liver or herpes-related fatigue [47].
Yashtimadhu (Glycyrrhiza glabra)
The compound glycyrrhizin in licorice root exhibits antiviral activity against both HSV-1 and HSV-2. It also reduces inflammation and soothes mucosal ulcers, which makes it useful during flare-ups and oral herpes [48].
Neem (Azadirachta indica)
Used extensively in chickenpox, neem is antimicrobial, antiviral, and antipyretic. Its bitter action pacifies Pitta and Rakta disorders, making it ideal for skin eruptions, itching, and post-viral scarring [49].
Haridra (Curcuma longa)
Curcumin has proven antiviral actions, including the suppression of HSV replication. It works best when inflammation, heat, and redness are dominant symptoms. Turmeric also helps clear pigmentary changes after pox lesions heal [50].
Kalmegh (Andrographis paniculata)
An immune-boosting herb with specific activity against flu, HSV, and even dengue viruses. It reduces viral load, improves T-cell activity, and supports fast recovery from fevers and latent viral symptoms like body ache and swelling [51].
Ashwagandha (Withania somnifera)
When the nervous system and immune system are exhausted post-infection, Ashwagandha offers neuroprotection, hormone balance, and improved mitochondrial health. It’s used especially in chronic herpes patients who feel drained or anxious [52].
Mineral-Based Ayurvedic Compounds with Antiviral and Rejuvenating Actions
This sulfur-based formulation is a staple in herpes treatment. It purifies the skin, improves immune memory, and heals recurrent ulcers. Unlike synthetic antivirals, Gandhak Rasayan also supports gut microbiota and detoxification [53].
Used in neuralgic symptoms, Abhrak is suited for post-herpetic nerve pain or CMV affecting brain tissue. It enhances Majja Dhatu, supports brain-immune crosstalk, and helps reverse viral damage at the cellular level [54].
This combination of copper, iron, and gold helps restore vitality after chronic infections. It is used in weak patients with anemia, post-fever debility, and in CMV cases with fatigue and low appetite [55].
This formulation of zinc, lead, and tin calxes is potent for managing genital herpes, infertility, and hormonal imbalance. It works on the Shukra Dhatu and is useful in male and female reproductive tract infections [56].
Silver calx is cooling and antimicrobial. It is ideal for burning lesions, oral sores, and mucosal ulcers caused by HSV-1 or chickenpox. It supports epithelial repair and reduces pain and irritation [57].
Used under supervision, Tamra Bhasma helps remove stubborn toxins and viruses hiding in the liver and blood. It improves enzyme function and acts as a potent antiviral when Rakta Dushti is involved [58].
A premium Rasayana used in chronic, latent viral infections with autoimmunity, low immunity, or emotional stress. Swarna Bhasma helps rebuild Ojas (vitality), supports T-cell activation, and stabilizes immunity in patients with recurrent herpes outbreaks or co-infections.
Master Polyherbal Formulation
This rare yet powerful Rasayana, mentioned in ancient texts, translates to “the rejuvenative that eliminates disease.” It contains a synergistic blend of antiviral, immuno-adaptive, and anti-inflammatory herbs like Amalaki, Guduchi, Haritaki, and Shatavari processed in decoctions and ghee. It is used in difficult viral infections like HSV, CMV, EBV, and even HIV, when multiple Dhatus are affected. It is especially suitable in patients with deep fatigue, emotional disturbance, weight loss, or multiple relapses.
Personalized Prescription: The Ayurvedic Difference
Unlike modern antivirals, which offer symptomatic suppression, Ayurvedic treatment is built on personalized diagnostics. Every antiviral Rasayana is matched to a person’s doshic imbalance, chronicity, immunity, and mental-emotional health.
For example:
- A Pitta-dominant patient with hot sores, ulcers, and high irritability may need Neem, Haridra, and Rajata Bhasma.
- A Vata-predominant patient with nerve pain, anxiety, and fatigue may benefit more from Abhrak Bhasma, Ashwagandha, and Guduchi.
- A Kapha-dominant case with thick lesions, lethargy, or mucus dominance may require Trikatu, Kalmegh, and Tamra Bhasma.
Formulations are not fixed—they’re changed every few weeks depending on response, recurrence, lab parameters, and Dhatu improvement.
When to Consider These Herbs and Minerals
Ayurvedic antiviral therapy can be initiated:
- After stopping synthetic antivirals
- During herpes outbreaks
- In asymptomatic patients with latent HSV/CMV (PCR positive)
- For post-herpes nerve pain, emotional fatigue, or fertility issues
- When modern medicine fails to clear viral DNA or prevent recurrence
Patients should not self-medicate. These formulations require clinical monitoring, especially herbo-mineral combinations like Tamra or Swarna Bhasma.
Long-Term Complications if Left Untreated
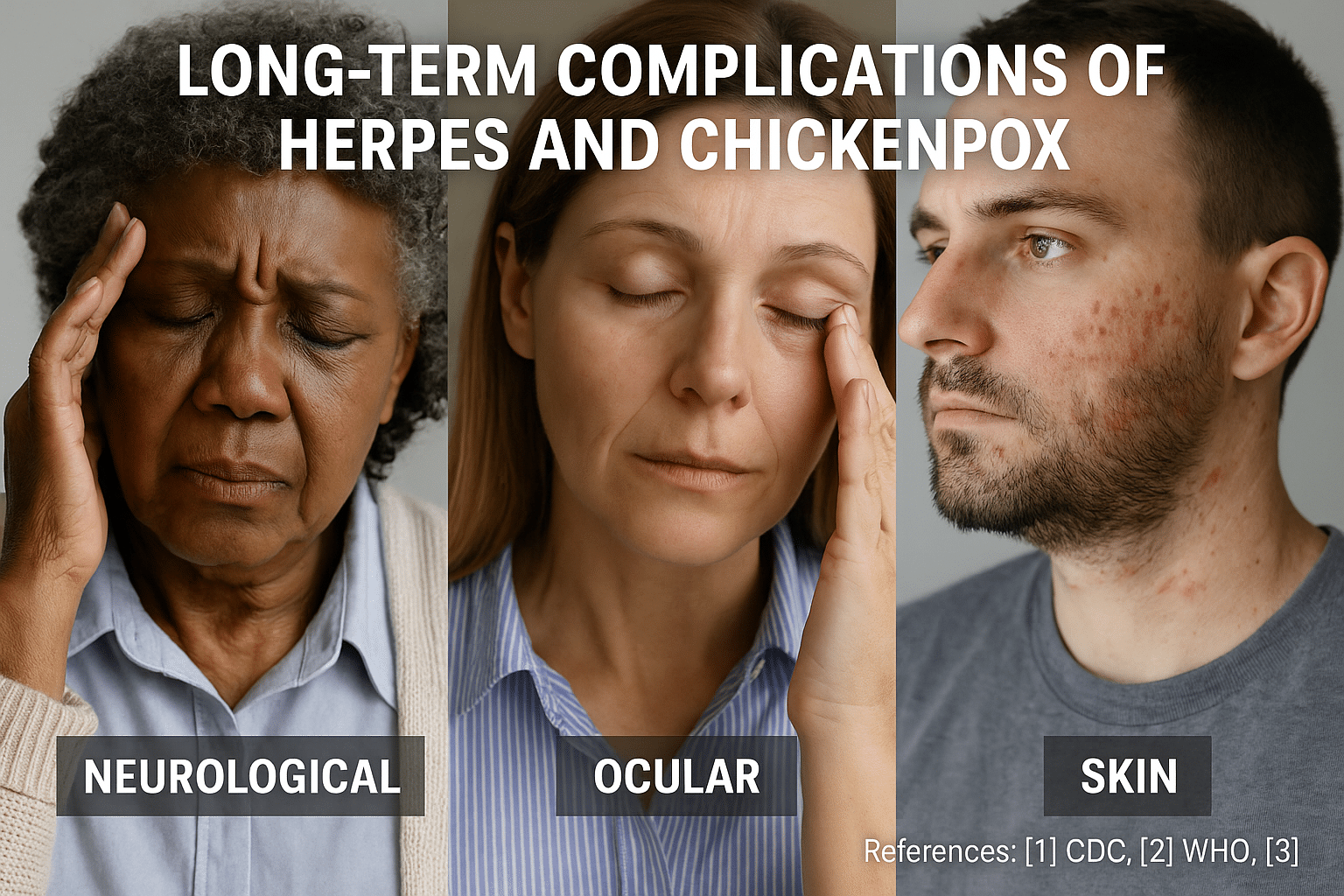
Most people view herpes and chickenpox as minor viral infections with temporary symptoms. However, beneath the surface, these viruses have the potential to trigger deep, long-lasting health issues—especially if not addressed holistically. When left untreated or suppressed, both Herpes Simplex Virus (HSV) and Varicella-Zoster Virus (VZV) can persist in the body and resurface in more severe forms, often compromising the immune system, nervous tissues, and even reproductive health.
Herpes Complications: Beyond the Skin
Nerve damage and chronic pain:
Herpes simplex virus (especially HSV-2) establishes latency in the sacral dorsal root ganglia, the cluster of nerves near the spinal cord. Over time, this can cause chronic pelvic neuralgia, burning sensations, and nerve hypersensitivity in the genital region. This condition often remains undiagnosed but is responsible for sexual discomfort, insomnia, and reduced quality of life [59].
Herpetic meningitis and encephalitis:
In rare but serious cases, herpes can invade the central nervous system, resulting in aseptic meningitis or viral encephalitis. HSV-1 is the leading cause of sporadic encephalitis globally. Survivors may experience long-term cognitive impairment, seizures, and memory issues [60].
Eye damage (Herpes keratitis):
When HSV reactivates in the ophthalmic branch of the trigeminal nerve, it can affect the eyes. This may cause herpes keratitis, leading to scarring of the cornea, vision loss, and even blindness if not treated promptly [61].
Reproductive health and fertility issues:
Chronic HSV-2 infection is linked to uterine inflammation, pelvic inflammatory disease, and impaired sperm motility. In women, it increases the risk of ectopic pregnancy, infertility, and spontaneous abortion during early pregnancy [62].
HIV and STD co-transmission:
Herpes significantly increases the risk of acquiring and transmitting HIV. The virus weakens mucosal barriers and creates microscopic lesions, providing easy entry points for other pathogens [63]. It also interacts with Human Papillomavirus (HPV), enhancing the progression of cervical dysplasia and cancer in women.
Herpes during pregnancy:
Primary herpes infection during late pregnancy poses a high risk of neonatal herpes, which can lead to neurological damage, organ failure, or death in newborns. Cesarean delivery is often recommended if lesions are present near delivery [64].
Chickenpox Complications: The Shingles Legacy
Shingles and post-herpetic neuralgia (PHN):
After recovery from chickenpox, the VZV remains dormant in the nerve roots. Years or decades later, it can reactivate as Herpes Zoster (shingles), causing a painful, blistering rash on one side of the body. The pain may linger even after rash resolution—a condition known as post-herpetic neuralgia, which can be debilitating and lifelong in older individuals [65].
Ophthalmic shingles and vision loss:
When the virus affects the ophthalmic division of the trigeminal nerve, it can cause herpes zoster ophthalmicus, leading to inflammation of the cornea, glaucoma, or permanent visual impairment [66].
Neurological disorders:
Varicella-Zoster reactivation can trigger stroke, cerebellitis, vasculitis, and Guillain-Barré Syndrome. It can even mimic multiple sclerosis in imaging studies. These are increasingly being reported in younger individuals due to immune dysregulation or poor viral clearance [67].
Disseminated VZV in immune-compromised patients:
In people with weakened immunity, such as cancer patients or transplant recipients, VZV may spread to internal organs like the lungs, liver, or brain, leading to multi-organ damage and high mortality [68].
Ayurvedic Insight into Long-Term Viral Load
Ayurveda identifies these post-infection complications as a result of viral latency within Majja Dhatu (nervous tissue) and Shukra Dhatu (reproductive tissue). When the virus remains hidden but active in the deeper tissues, it leads to Dhatukshaya (tissue depletion), Ojakshaya (immunodeficiency), and Vyadhi Sankara (multi-systemic disease fusion).
Unresolved infections block Srotas (channels) and disturb the balance of Vata-Pitta, which drives neurological, immune, and inflammatory pathways. This often manifests as:
- Nerve pain, burning, and fatigue – a classical sign of Vata-Rakta dushti
- Infertility, libido issues, and miscarriage – linked to Shukra Kshaya
- Immune collapse with co-infections – due to Ojas depletion
- Autoimmune-like relapses – where reactivation mimics diseases like lupus or fibromyalgia
Frequently Asked Questions
What is the main difference between herpes and chickenpox?
Chickenpox is an acute illness caused by the varicella-zoster virus (VZV), usually affecting children. Herpes is a lifelong viral infection caused by herpes simplex virus (HSV-1 or HSV-2). Chickenpox spreads through respiratory droplets and causes widespread itchy rashes, while herpes spreads through direct skin or mucous contact and leads to localized blisters most commonly oral or genital.
Can herpes spread through the air like chickenpox?
No. Chickenpox is airborne and highly contagious, particularly during coughing or sneezing. Herpes is not airborne. It transmits via skin-to-skin contact, kissing, or sexual activity—even without visible sores through a phenomenon known as asymptomatic viral shedding.
Do both viruses stay in the body permanently?
Yes. Both HSV and VZV establish latency in the sensory ganglia of the nervous system. Chickenpox can reactivate later as shingles, while herpes reactivates more frequently as recurrent cold sores or genital outbreaks, especially during stress or weakened immunity.
Is herpes more dangerous than chickenpox?
Herpes can be more dangerous in the long term. It increases the risk of neonatal infections, HIV co-transmission, and chronic nerve pain. Chickenpox, though severe in adults and immunocompromised patients, usually resolves in children without recurrence. HSV, especially HSV-2, is more persistent and psychologically distressing due to its recurrence and social stigma.
What are the key symptoms of herpes vs chickenpox?
Chickenpox presents with:
– Fever, fatigue
– Itchy, red rash that spreads to the entire body
– Rash matures into fluid-filled blisters
Herpes (HSV-1/2) symptoms:
– Burning, tingling followed by blisters around lips or genitals
– Ulcers that crust and heal slowly
– Pain during urination or intercourse (HSV-2)
Can you get both herpes and chickenpox together?
Yes, though uncommon. Individuals with weakened immunity (e.g., HIV-positive or post-COVID) may harbor multiple herpesviruses like VZV, HSV-1/2, CMV, or EBV simultaneously. Ayurveda refers to this condition as Vyadhi-Sankara a compounded disease state where multiple pathogenic energies disturb Ojas and Dhatus.
How are chickenpox and herpes diagnosed?
Chickenpox is usually diagnosed clinically. For herpes, DNA PCR of swabs or blood is the gold standard. HSV IgM is often unreliable and not recommended. Viral culture or Tzanck smear may also be used in some settings.
Is there a vaccine for both?
Yes, chickenpox and shingles vaccines are well-established. There is no FDA-approved herpes vaccine for humans yet, though clinical trials are ongoing. Ayurvedic preventive Rasayanas are often used to boost host resistance in recurring cases.
Can herpes be spread through a cut or blood?
Yes. Although less common, HSV can enter through microabrasions or cuts, especially during sexual contact. Even invisible breaks in mucosal tissue are sufficient for transmission, making it highly contagious in intimate settings.
Can Ayurveda cure herpes permanently?
Ayurveda aims for complete viral clearance, not just suppression. Using Rasayana therapy, herbo-mineral preparations like Swarna Bhasma, Vyadhiharan Rasayan, Guduchi Satva, and antiviral herbs like Neem, Bhumyamalaki, and Kalmegh, Ayurveda targets latent viral load in Majja Dhatu (nervous tissue). Treatment is personalized according to Prakriti, viral strain, immunity, and co-infections. Panchakarma is optional but can help detoxify deeper tissues when needed.
Reference
Note:
Every reference listed here has been carefully selected for accuracy, clinical relevance, and traceability. Ayurvedic formulations are cited directly from classical medical texts (Charaka Samhita, Sushruta Samhita, Bhavaprakasha, etc.) along with specific verse numbers and chapters. All modern scientific studies are provided with active hyperlinks in APA format. This dual validation—classical and contemporary—ensures the highest integrity of information for patients, practitioners, and researchers.
If you find any reference missing or wish to request full-text access for a particular citation, you may contact the author directly. Our goal is to maintain complete transparency and academic rigor.
All Source
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Bäcker, H., & Naher, H. (1997). PCR detection of herpes simplex virus DNA in clinical specimens. Clinical Microbiology and Infection, 3(3), 269–274. https://doi.org/10.1111/j.1469-0691.1997.tb00385.x ↩
- Charaka Samhita, Chikitsa Sthana 1/3–30. Commentary by Chakrapani Datta. (Ed. Dr. P.V. Sharma). Chaukhambha Orientalia. ↩
- Sushruta Samhita, Uttara Tantra 61/15–22. (Ed. Kaviraj Ambikadutta Shastri). Chaukhambha Sanskrit Sansthan. ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Bäcker, H., & Naher, H. (1997). PCR detection of herpes simplex virus DNA in clinical specimens. Clinical Microbiology and Infection, 3(3), 269–274. https://doi.org/10.1111/j.1469-0691.1997.tb00385.x ↩
- Charaka Samhita, Chikitsa Sthana 1/3–30. Commentary by Chakrapani Datta. (Ed. Dr. P.V. Sharma). Chaukhambha Orientalia. ↩
- Bhavaprakasha Nighantu. Guduchyadi Varga – Guduchi, Nimba, Bhumyamalaki. (Ed. K.C. Chunekar, Commentary by G.S. Pandey). Chaukhambha Bharati Academy. ↩
- Singh, G., Kumar, A., & Agrawal, P. (2018). Antiviral activity of Bhumyamalaki (Phyllanthus niruri). Pharmacognosy Reviews, 12(24), 74–80. https://doi.org/10.4103/phrev.phrev_5_18 ↩
- Sharma, A., & Chandola, H. M. (2011). Clinical efficacy of Rasayana therapy in the management of chronic viral infections. AYU, 32(4), 436. https://doi.org/10.4103/0974-8520.96130 ↩
- Bhavaprakasha Nighantu. Guduchyadi Varga – Guduchi, Nimba, Bhumyamalaki. (Ed. K.C. Chunekar, Commentary by G.S. Pandey). Chaukhambha Bharati Academy. ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Bäcker, H., & Naher, H. (1997). PCR detection of herpes simplex virus DNA in clinical specimens. Clinical Microbiology and Infection, 3(3), 269–274. https://doi.org/10.1111/j.1469-0691.1997.tb00385.x ↩
- Charaka Samhita, Chikitsa Sthana 1/3–30. Commentary by Chakrapani Datta. (Ed. Dr. P.V. Sharma). Chaukhambha Orientalia. ↩
- Sushruta Samhita, Uttara Tantra 61/15–22. (Ed. Kaviraj Ambikadutta Shastri). Chaukhambha Sanskrit Sansthan. ↩
- Bhavaprakasha Nighantu. Guduchyadi Varga – Guduchi, Nimba, Bhumyamalaki. (Ed. K.C. Chunekar, Commentary by G.S. Pandey). Chaukhambha Bharati Academy. ↩
- Singh, R., Geetanjali, & Meena, H. (2020). Swarna bhasma: An ancient nanomedicine for cancer and chronic diseases. Journal of Ayurveda and Integrative Medicine, 11(3), 293–300. https://doi.org/10.1016/j.jaim.2019.06.005 ↩
- Sharma, A., & Chandola, H. M. (2011). Clinical efficacy of Rasayana therapy in the management of chronic viral infections. AYU, 32(4), 436. https://doi.org/10.4103/0974-8520.96130 ↩
- Singh, G., Kumar, A., & Agrawal, P. (2018). Antiviral activity of Bhumyamalaki (Phyllanthus niruri). Pharmacognosy Reviews, 12(24), 74–80. https://doi.org/10.4103/phrev.phrev_5_18 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩
- Xu, F., Sternberg, M. R., Kottiri, B. J., McQuillan, G. M., Lee, F. K., Nahmias, A. J., & Markowitz, L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296(8), 964–973. https://doi.org/10.1001/jama.296.8.964 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Arvin, A. M. (2001). Varicella-zoster virus. Clinical Microbiology Reviews, 14(3), 560–573. https://doi.org/10.1128/CMR.14.3.560-573.2001 ↩
- Looker, K. J., Magaret, A. S., May, M. T., Turner, K. M., Vickerman, P., & Newman, L. M. (2015). Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE, 10(10), e0140765. https://doi.org/10.1371/journal.pone.0140765 ↩
- James, S. H., & Kimberlin, D. W. (2015). Neonatal herpes simplex virus infection. Infectious Disease Clinics of North America, 29(3), 391–400. https://doi.org/10.1016/j.idc.2015.05.003 ↩
- Wald, A., & Link, K. (2002). Risk of human immunodeficiency virus infection in herpes simplex virus type 2–seropositive persons: A meta-analysis. The Journal of Infectious Diseases, 185(1), 45–52. https://doi.org/10.1086/338231 ↩