- Primary Modes of Transmission
- Viral Shedding and Infectious Period
- Role of Immunity and Host Response
- What Triggers- Environmental and Lifestyle Triggers
- Lesser-Known but Medically Significant Triggers
- Why Some People Get Infected While Others Don’t
- Why Herpes Reactivates – The Latency Puzzle
- Sexual Contact and Multi-Virus Transmission
- Hidden Routes – From Mother to Child and Organ Donation
- Tattoos, Piercings, and Shared Grooming Tools
- Surgical Instruments and Hospital-Acquired Spread
- Blood Transfusions and Transplanted Organs
- Laboratory Accidents and Contaminated Instruments
- Blood Transfusion, Organ Transplant, and IVF Transmission
- Frequently Asked Questions
- References
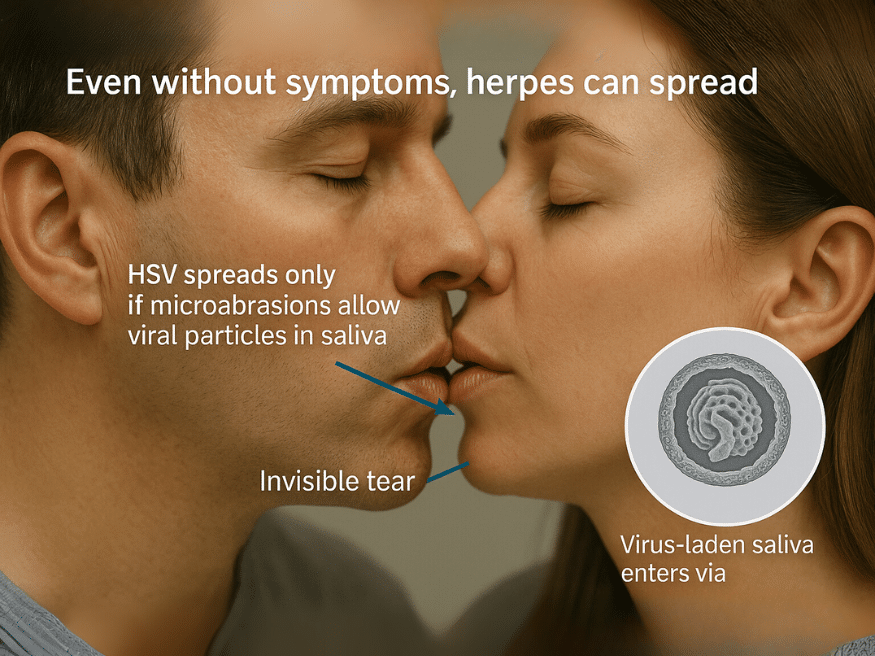
Herpes simplex virus (HSV) is a highly contagious viral infection that spreads primarily through blood contact and saliva exposure in the presence of microabrasions or invisible sores. Understanding how herpes spreads is crucial for preventing silent transmission, which often occurs even when symptoms are absent. The virus has two main strains—HSV-1, often linked with oral infections, and HSV-2, more associated with genital infections—both capable of establishing latency in nerve tissue and reactivating throughout life [1, 2].
Contrary to common belief, the herpes virus does not survive long on surfaces and does not pass easily through intact skin. Instead, it enters the body through microscopic skin injuries or mucosal abrasions. Even simple acts such as sharing a glass, toothbrush, or kissing can lead to transmission if these invisible breaches exist—because saliva may carry the virus in fine, suspended viral particles, especially during asymptomatic shedding [3, 4]. In such cases, herpes viruses (and other members of the herpes family like CMV, EBV, HHV-6, or even HIV) can enter the bloodstream or mucosa via damaged tissue [5].
This understanding challenges the oversimplified notion that kissing or sharing objects is “safe” in all cases. Kissing alone can be enough to transmit herpes or other blood-borne viruses if the host carries the virus in saliva and the recipient has microlesions—even if they’re not visible to the eye [6, 7]. As a result, most primary infections happen without the individual’s knowledge of exposure, often during childhood or intimate relationships.
From an Ayurvedic perspective, such transmission aligns with the Krimi theory, where pathogenic Dravya Krimi (minute fluid-based organisms) penetrate disturbed Srotas (body channels) when protective immunity (Ojas) is weakened [8]. Viral invasion is considered more likely in individuals with Dosha imbalance, Srotodushti (obstructed channels), and Rakta Dhatu Dushti (blood tissue vitiation), conditions that make the host more susceptible to disease even without direct lesion contact.
This article presents a comprehensive review of both classical and emerging insights into how herpes truly spreads—with in-depth explanation of blood-based, mucosal, and salivary pathways—bridging modern virology with Ayurvedic channel theory for a deeper, clinically relevant understanding.
Primary Modes of Transmission
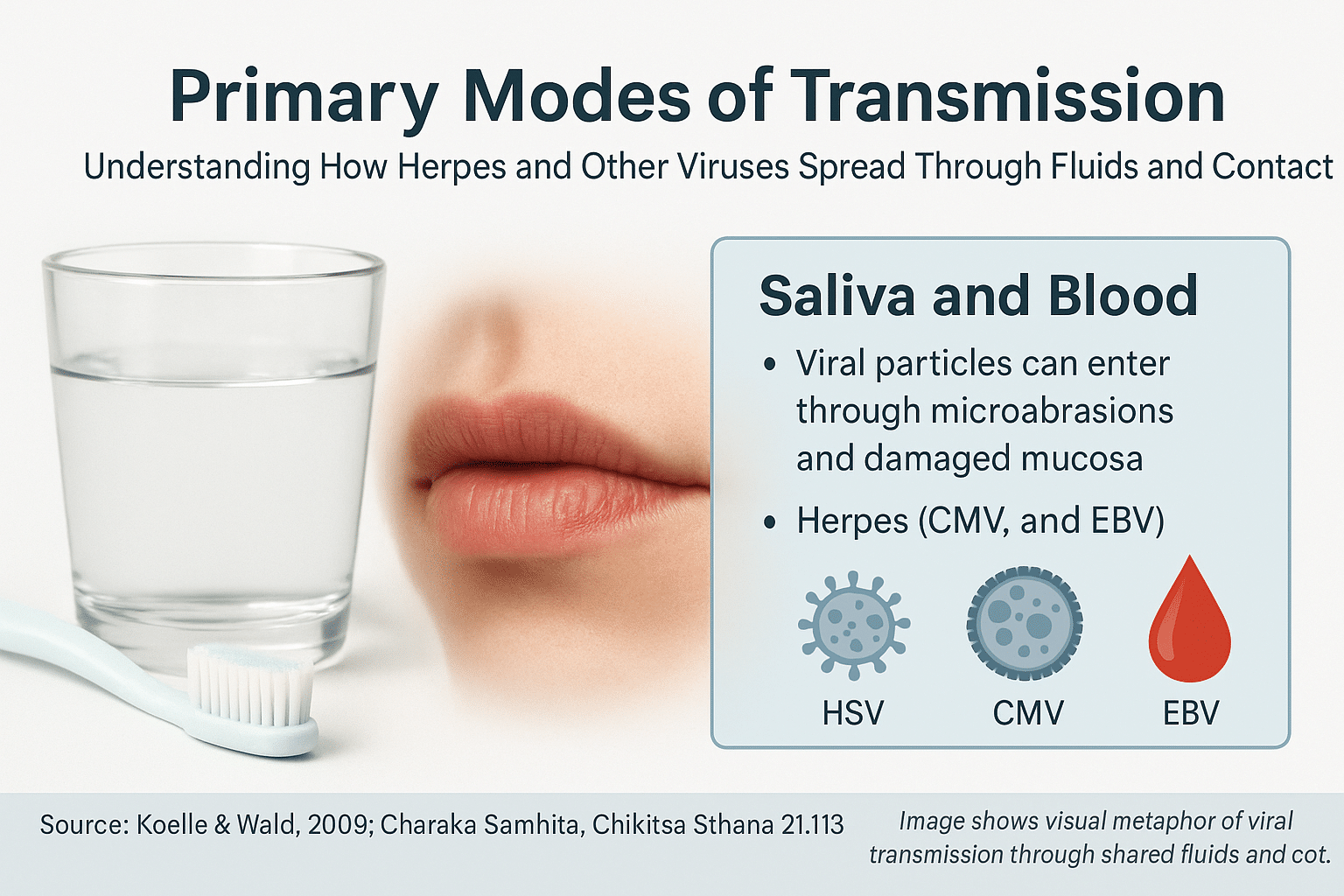
Herpes viruses do not spread through casual air exposure, handshakes, or distant contact. Instead, the transmission of herpes—and other viruses from the same family—occurs when infected saliva or sexual fluids containing viral particles come in contact with microabrasions, damaged mucosa, or compromised skin. These nearly invisible injuries act as portals, allowing the virus to penetrate deeper tissues and initiate infection.
Importantly, saliva and sexual fluids do not carry just one virus. If the infected individual harbors HSV-1, HSV-2, CMV, EBV, HHV-6, or even HIV, all of these can be transferred simultaneously through a single contact event. Whether the host becomes infected with one, several, or none of these viruses depends on their immune strength, mucosal integrity, and internal susceptibility.
1. Skin-to-Skin Contact with Microabrasions
Most people assume herpes spreads only during visible outbreaks. However, invisible skin cuts or abrasions—caused by shaving, brushing, dry weather, or even tight clothing—can allow viral particles to enter. When infected saliva or sexual fluids touch these micro-wounds, the virus bypasses surface defenses and reaches the inner layers of skin or mucosa.
2.Saliva-Based Transmission via Kissing or Shared Objects
Saliva is a potent yet underrecognized carrier of multiple viruses, including HSV-1, HSV-2, CMV, EBV, HHV-6, and even HIV under certain conditions. While herpes transmission is the primary concern in most cases, the truth is that all viruses present in an infected individual’s saliva can be transferred—especially when the receiving person has mucosal abrasions or a weakened immune system.
During asymptomatic viral shedding, even in the absence of visible sores, viral particles are suspended in saliva in microscopic quantities. If that saliva comes in contact with invisible cuts, dry lips, inflamed gums, or irritated oral tissue, the viruses can penetrate and begin replication. Which viruses actually establish infection depends on the host’s immunity, strength of Ojas (vital life energy), and the balance of Doshas affecting tissue resistance.
Example 1: The Kiss
When an infected person kisses someone, they may unknowingly transmit not just HSV-1, but also CMV, EBV, or other co-infections harbored in their saliva. If the recipient has cracked lips, bleeding gums, or mild oral inflammation, these viruses can enter the bloodstream or deeper tissues. Whether one, multiple, or none of these viruses establish infection depends on the immune defense at that moment. A person with strong local immunity (oral mucosal IgA or high Ojas) may resist infection, while someone with poor gut health, chronic stress, or vitamin deficiency may absorb and retain multiple viruses at once.
Example 2: Sharing a Toothbrush or Utensil
The warm, moist bristles of a toothbrush or the rim of a cup can preserve viral particles long enough for transmission. If a second person uses these objects—especially after brushing hard, flossing, or experiencing gum bleeding—they create a direct viral entry point. Again, the outcome depends on the immune landscape: someone may develop only HSV-1, while another may simultaneously contract HSV-1 and EBV, depending on their tissue susceptibility and Rakta Dhatu status.
Example 3: Drinking from the Same Bottle
In shared settings like families, hostels, or relationships, people often drink from the same bottle. The risk is underestimated. If the infected person’s saliva contains a cocktail of latent or active viruses, and the recipient has oral dryness, canker sores, or even digestive inflammation, these viruses may enter the system and lodge in compatible tissues, like the salivary glands (for CMV) or nerve ganglia (for HSV).
In Ayurveda, this transmission mirrors the invasion of Drava Krimi (fluid-borne pathogens) through vulnerable Srotas (microchannels). The likelihood of viral colonization increases when the host’s Agni (metabolic fire) is low, Rakta and Mamsa Dhatus (blood and muscle tissues) are weakened, and Ojas is compromised. This explains why two individuals exposed to the same virus may respond differently—one becomes symptomatic with multiple viruses; the other remains asymptomatic or partially affected [9].
This multi-virus transfer through saliva also explains complex clinical patterns seen today—where patients test positive for HSV, CMV, EBV, and HHV-6 simultaneously, often with no memory of high-risk exposure. What began as a harmless kiss, or a shared glass, becomes a silent entry point for multiple persistent viruses, governed not just by exposure, but by inner terrain.
Viral Survival Times on Surfaces (Rare but Possible)
Scientific studies show that:
- Herpes simplex virus (HSV-1) can survive for up to 2–4 hours on dry surfaces like plastic or glass, and up to 8 hours in moist conditions such as inside a used cup, on a lip balm, or within toothbrush bristles [10].
- CMV (Cytomegalovirus), another saliva-borne herpesvirus, can remain infectious on surfaces like glass or plastic for up to 6 hours, and in damp environments (like wet towels or utensils) for more than 24 hours [11].
- EBV (Epstein–Barr virus) survives for shorter periods on dry surfaces but may remain viable in saliva-containing fluids for a few hours [12].
What this means is that if a glass or spoon was used within the last 1–4 hours by someone shedding HSV or other viruses, and then used by another person with oral cuts, sore gums, or mucosal dryness, the infection may successfully transfer—even in the absence of direct contact.
Real-Life Examples
- Toothbrush sharing: HSV can remain viable in toothbrush bristles for up to 48 hours in moist conditions, especially if stored in a closed container. Saliva-laden bristles can transfer the virus directly into gum tissues weakened by brushing or preexisting inflammation.
- Shared lip balm or lipstick: The virus can stay infectious for several hours on cosmetic surfaces, especially waxy or oil-based textures that preserve moisture. If cracked lips or minor wounds are present, this creates an ideal transmission path.
- Drinking from the same bottle: Cold beverages or sugary drinks provide moisture and sugar, which may preserve the virus temporarily. Combined with frequent use and backwash, these objects may pass multiple viruses to an unsuspecting host.
Role of Immunity and Host Response
Not everyone exposed will get infected. Whether or not infection takes hold depends on:
- Mucosal integrity: Dry lips, gingivitis, ulcers, or cracked skin increase risk
- Systemic immunity: Individuals with low immunity, recent illness, nutritional deficiencies, or high stress are more likely to absorb and retain the virus
- Viral load in saliva: Higher shedding correlates with increased transmission risk
Therefore, the same exposure event may lead to different outcomes. One person may develop HSV-1, another CMV, and another may stay asymptomatic—all based on immune resilience and inner terrain.
3. Sexual Contact and Mixed Fluids
Genital herpes (mostly HSV-2) spreads through oral, vaginal, or anal sex. What most people don’t realize is that sexual fluids almost always contain microscopic amounts of blood, especially during menstruation, vigorous intercourse, or in the presence of inflammation or ulcers. These fluids may carry multiple viruses simultaneously, including HSV, CMV, EBV, and HIV.
If the receiving partner has even minor mucosal abrasions, infection can occur—regardless of condom use, as the virus may be present on skin not covered by protection.
4. Oral-Genital and Oral-Anal Transmission
Oral sex can transmit HSV-1 to the genital region, and vice versa. When someone with oral herpes performs oral sex on a partner with microabrasions, the saliva introduces the virus directly into the genital or anal mucosa. The same applies for oral-anal contact (rimming), where tissue fragility increases the risk of multiple virus transmission.
5. Bloodborne Transmission via Microtrauma
While HSV is not classified as a bloodborne virus, it can enter the bloodstream when saliva or sexual fluid contaminated with blood contacts small wounds. This can happen during dental work, kissing with bleeding gums, or sharing razors. If microvascular trauma is present, the virus can be absorbed systemically. This route, though less common, is especially dangerous when co-infections like HIV or CMV are also present.
6. Vertical Transmission (Mother to Child)
During vaginal delivery, herpes and other viruses can pass from mother to infant through exposure to blood and vaginal secretions. If the mother is actively shedding HSV-2 at birth, the newborn is at risk for neonatal herpes, which may affect the brain, liver, or lungs and can be fatal if untreated.
Role of Immunity and Host Response
Not every exposure leads to infection. Whether or not a virus establishes itself depends on:
- Mucosal integrity: Dry lips, bleeding gums, ulcers, and inflammation create vulnerability
- Systemic immunity: Stress, nutritional deficiency, recent illness, or underlying disease reduce the body’s ability to neutralize viruses
- Viral load in fluids: A higher concentration of virus increases the probability of successful entry
Thus, two people exposed to the same virus under the same conditions may have completely different outcomes. One may develop HSV, another CMV, and another none at all—depending on immune resilience and internal terrain.
Ayurvedic Interpretation
In Ayurveda, these forms of viral entry are viewed through the lens of Drava Krimi (fluid-borne pathogens) and Bahya Krimi (external invaders). Viruses enter weakened Srotas (body channels) when Rakta Dhatu (blood), Mamsa Dhatu (muscle tissue), and Ojas (vital life force) are depleted. Preventive strategies include daily Dantadhavana (herbal brushing), Gandusha (oil pulling), and Kavala (herbal gargling) to fortify the oral mucosa and build internal resistance to such silent invasions.
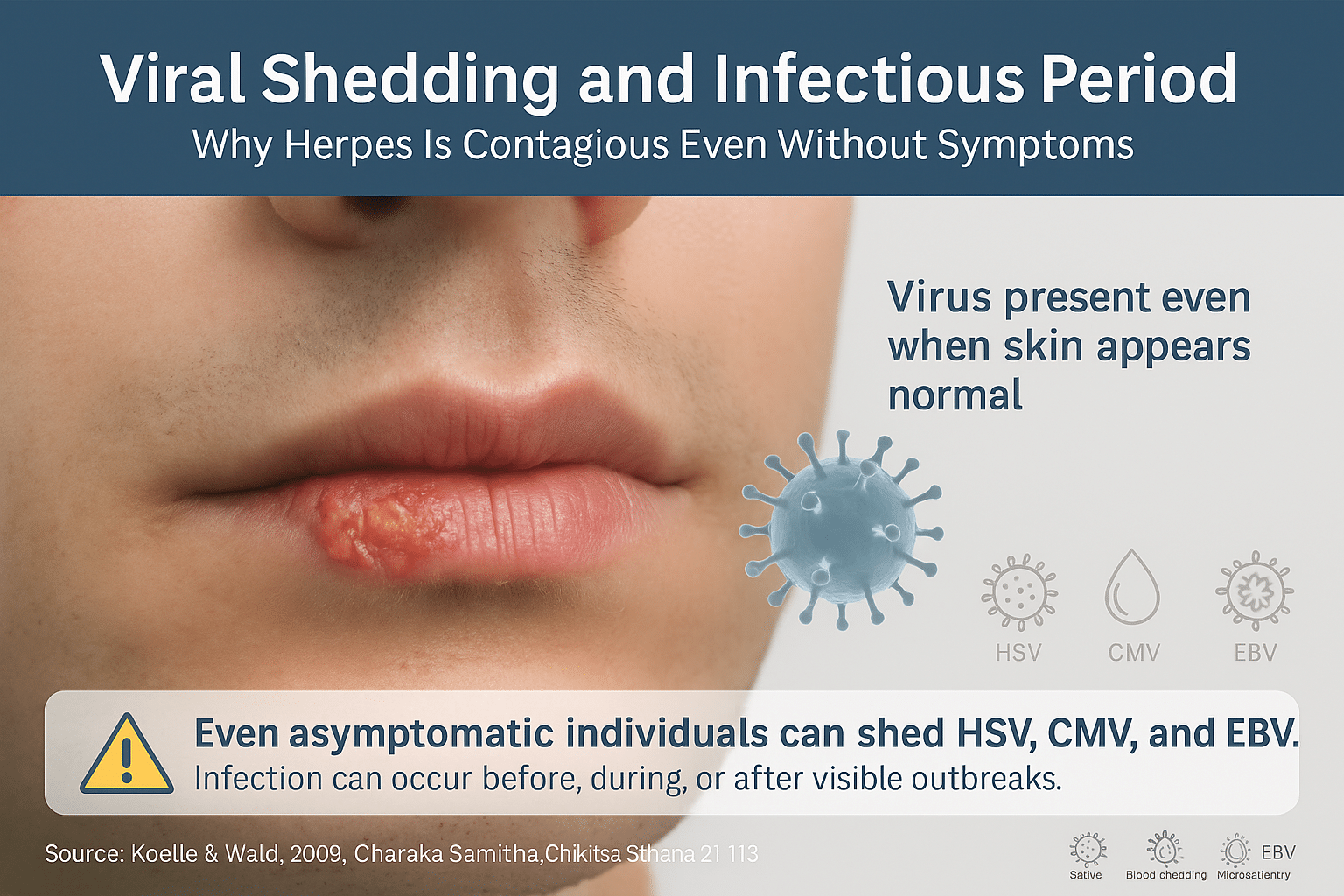
Herpes Is Contagious Even Without Symptoms
One of the most overlooked aspects of herpes transmission is viral shedding, the process by which herpes virus particles exit the nerve ganglia and appear on the skin or mucosa—even in the absence of visible lesions. This is known as asymptomatic shedding and is responsible for a large percentage of new herpes infections globally. Unlike many viral infections that are contagious only during flare-ups, herpes can be silently passed from person to person during symptom-free periods [13].
How and When Shedding Occurs
After initial infection, HSV becomes dormant in the dorsal root ganglia, a cluster of nerves near the spinal cord. When reactivated—triggered by factors like stress, fever, hormonal shifts, UV exposure, or lowered immunity—it travels back to the skin surface and begins to replicate. Shedding can begin before any symptoms arise, continue during an outbreak, and persist even after visible healing has occurred. Studies show that individuals with recurrent HSV may shed the virus asymptomatically on 10–20% of days in a given month [14].
Duration of Viral Shedding
The shedding period varies based on multiple factors:
- Primary infection: Shedding can last up to 3 weeks, even if symptoms are mild or absent.
- Recurrent infection: Shedding typically lasts 2–5 days, but may extend longer in immunocompromised individuals.
- HSV strain: HSV-2 tends to shed more frequently and for longer periods in the genital area compared to HSV-1.
This variability means that even when no cold sores or genital lesions are present, the person may still be highly infectious [15].
Viral Shedding Through Saliva, Genital Fluids, and Tears
HSV is not confined to just skin. The virus is shed through saliva, semen, vaginal secretions, and even tears, depending on the site of infection and immune status.
- HSV-1 is commonly found in saliva and can be passed through kissing, sharing drinks, or oral sex—even with no visible sores.
- HSV-2 sheds through genital secretions and can be transmitted during intimate contact with infected mucosa or fluids, even with no obvious signs.
This makes herpes difficult to control through symptom monitoring alone [16].
Ayurvedic Insight: Subtle Shedding as Sukshma Krimi Vyapti
In Ayurveda, such persistent and invisible shedding is understood as “Sukshma Krimi Vyapti”—the subtle spread of microbial life through Srotas (body channels). Even when no external Lakshana (symptoms) are visible, Krimi activity continues within Rakta Dhatu (blood) and Mamsa Dhatu (muscle tissue). It indicates chronic Srotodushti (microchannel pathology) and depletion of Ojas, the body’s core immunity [17].
Hence, treatment must go beyond surface-level management. Ayurvedic therapy focuses on restoring balance in the channels, removing dormant viral presence, and rebuilding Ojas through Rasayana herbs, blood-cleansing preparations, and immune-stabilizing protocols.
Breaking the Cycle of Silent Spread
Because herpes sheds even when silent, many people unknowingly infect partners. Suppressive antiviral drugs can reduce—but not eliminate—this risk. In contrast, the Ayurvedic approach targets deep detoxification, Dhatu regeneration, and Srotas purification, aiming for total viral clearance.
Understanding viral shedding reframes the prevention strategy: it’s not just about avoiding visible outbreaks—it’s about acknowledging the risk of invisible contagion. For long-term protection, both partners need awareness, mucosal care, Ayurvedic support, and personalized preventive strategies.
Role of Immunity and Host Response
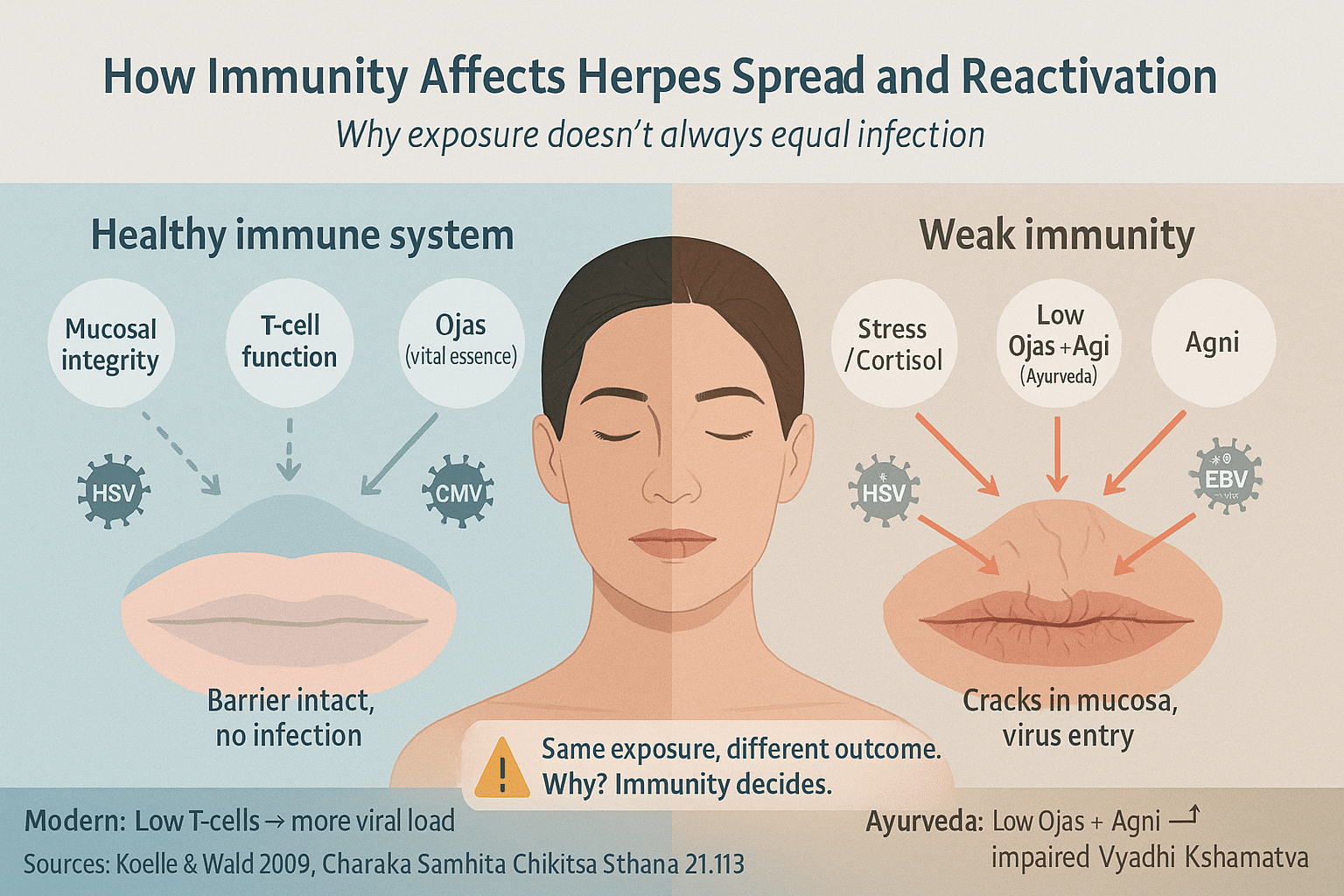
Why Not Everyone Gets Infected After Exposure
Herpes transmission is not a simple matter of contact alone. Exposure to the virus does not guarantee infection. Whether or not the virus takes hold—and whether it remains latent, becomes symptomatic, or is fully resisted—depends on the host’s immune status at the time of contact. This explains why some people develop herpes after a single exposure, while others may never test positive despite repeated encounters with an infected partner [18].
The virus relies on gaps in mucosal integrity, compromised immune surveillance, or weakened local defenses to initiate infection. If the skin or mucosal barrier is inflamed, abraded, or damaged—even at a microscopic level—the virus can enter and begin replication. However, a robust innate immune response may neutralize the virus early, preventing its entry into nerve ganglia and long-term latency [19].
Cellular Immunity: The First Line of Defense
After exposure, the body mounts an immune response involving T-cells, natural killer (NK) cells, and mucosal antibodies (IgA). These cellular defenders recognize viral proteins and attempt to clear the infection. Individuals with a well-regulated Th1/Th2 balance and strong antigen-specific T-cell responses are more likely to suppress HSV reactivation and reduce asymptomatic shedding. In contrast, low T-cell counts, chronic stress, or autoimmune dysfunctions are strongly associated with prolonged viral persistence and higher recurrence rates [20].
Factors That Weaken Host Defense
Multiple internal and external factors can impair immune efficiency, including:
- Chronic stress (increases cortisol, suppressing immunity)
- Sleep deprivation and irregular circadian rhythm
- Vitamin deficiencies (especially zinc, vitamin D, and B-complex)
- Gastrointestinal imbalances (e.g., leaky gut, dysbiosis)
- Overuse of antibiotics or steroids
- Uncontrolled diabetes, HIV, or autoimmune disorders
These factors not only lower immune function but also disrupt the body’s viral detection systems, allowing herpes to remain hidden or reactivate frequently [21].
Ayurvedic View: Ojas, Agni, and Vyadhi Kshamatva
In Ayurveda, immunity is not seen as a single system but as an expression of Ojas (vital essence), Agni (digestive and metabolic fire), and Vyadhi Kshamatva (disease resistance). When Agni is strong and Ojas is well-nourished, the body resists not just symptoms but the very establishment of the virus in its tissues. However, if Agni is impaired—due to poor digestion, mental stress, or wrong food combinations—then Ama (toxic residue) accumulates, blocking Srotas (channels) and weakening immune response [22].
Ayurvedic texts also explain that Rakta Dushti (impurity of blood) and Mamsa Dhatu Kshaya (muscle depletion) make the system more susceptible to Krimi (microbial) invasion. Thus, building tissue strength (Dhatu Poshana), restoring Agni, and rejuvenating Ojas are essential goals in herpes treatment.
Personalized Immunity Determines Disease Outcome
Not all immune responses are created equal. A person with Pitta–Rakta dominance may develop sharp burning blisters and frequent inflammation. A Vata-type patient may suffer more from nerve pain and post-herpetic complications, while a Kapha-dominant constitution may harbor latent viral load with minimal outward expression—making them silent carriers. Therefore, immunity is not just strong or weak—it is patterned, and this pattern determines how the virus behaves in each host.
Modern immunology is beginning to recognize these differences in cytokine profiles, immune gene expression, and tissue susceptibility, validating what Ayurveda has observed for centuries through Prakriti-based analysis [23].
What Triggers- Environmental and Lifestyle Triggers
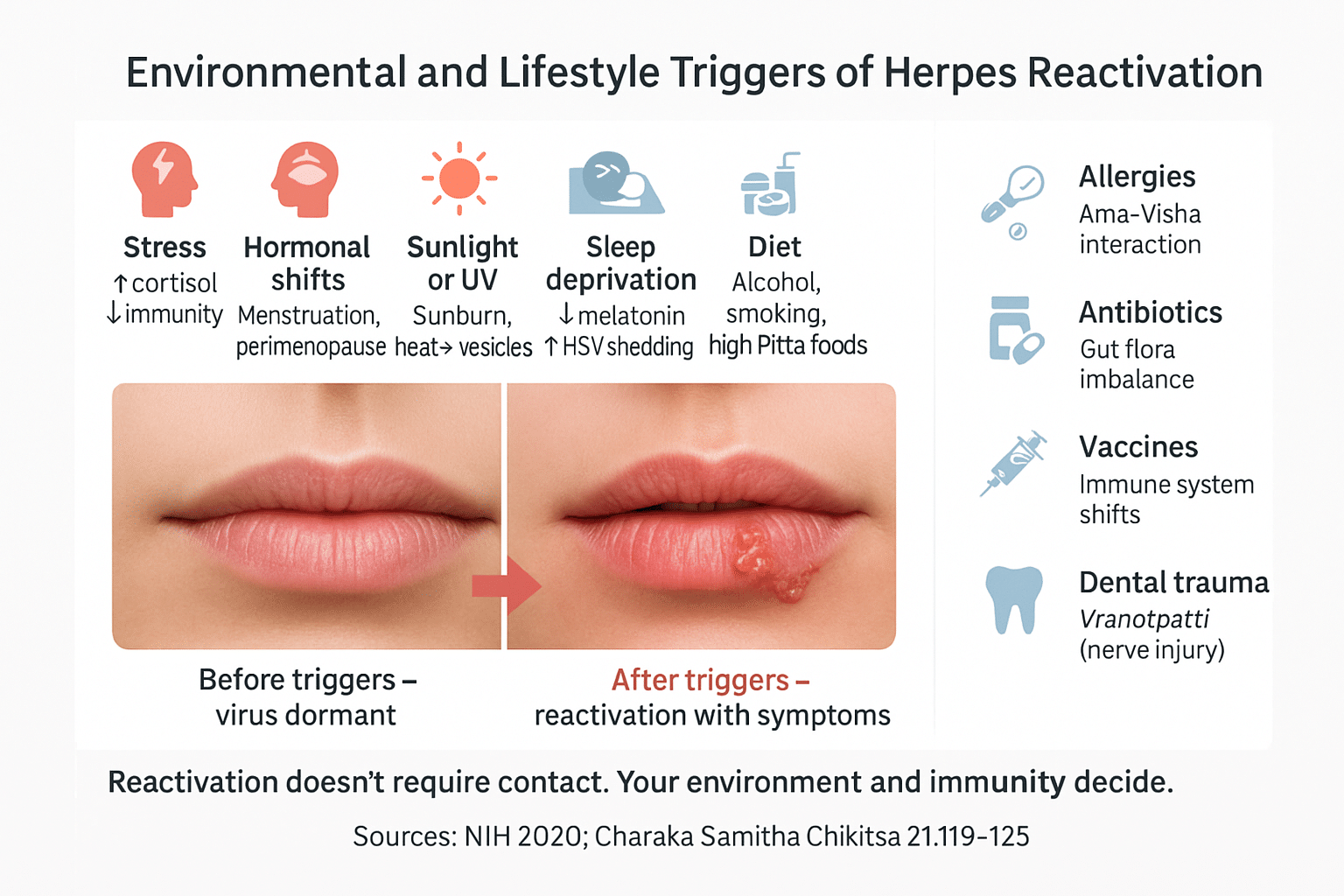
Why the Virus Reactivates Without Contact
Herpes reactivation is not always the result of direct physical contact. Instead, a wide range of internal and external stressors—environmental, immunological, and emotional—can awaken the virus from latency, even when no visible lesion is present. These triggers weaken the host’s immune terrain, creating an ideal environment for herpes viruses like HSV, CMV, EBV, and HHV-6 to resurface and begin shedding. Ayurveda describes this as Srotodushti (channel disturbance) and Ojakshaya (depletion of vital energy)—both key events in viral reactivation.
Stress and Emotional Strain
Among all known triggers, psychological and physical stress is the most dominant reactivation factor. Elevated cortisol levels reduce cytotoxic T-cell activity and mucosal immunity, allowing the virus to travel along nerves and reemerge at the surface. Chronic stress, trauma, or emotional suppression aggravate Vata and Pitta, further diminishing immunity. In Ayurveda, this is classified as Manasika Nidana, directly causing Dosha imbalance and Ojas loss.
Hormonal Shifts and Menstrual Changes
Herpes recurrence is strongly influenced by hormonal fluctuations, particularly in women. Menstruation, pregnancy, and perimenopausal transitions affect estrogen and progesterone levels, which modulate immune function. This leads to reduced mucosal resilience, especially in the vaginal and cervical regions. HSV-2 outbreaks often intensify during menstruation. Ayurveda addresses this through the lens of Yoni Vyapad (disorders of the reproductive tract) caused by Rakta Dushti and disturbed Artava Vaha Srotas.
Sleep Deprivation and Irregular Routines
Poor sleep, erratic work schedules, and travel-related jet lag disrupt melatonin production and circadian-regulated immunity. Research shows that individuals with poor sleep quality experience higher frequency and intensity of HSV outbreaks. In Ayurvedic terms, Nidra-Alpata (insufficient rest) impairs Agni, depletes Ojas, and increases Vata, destabilizing immune coordination.
Alcohol, Smoking, and Processed Sugar
These substances dampen both systemic and mucosal immunity. Alcohol and sugar promote inflammation and disturb the gut microbiome, while smoking damages respiratory and oral tissues. All three aggravate Pitta and Kapha, reduce tissue integrity, and generate Ama (toxic residue), contributing to reactivation and viral load increase. Ayurveda classifies these under Ahita Ahara-Vihara—diet and lifestyle factors that sabotage immune defense.
UV Exposure, Sunburn, and Heat
Direct sun exposure, especially on the lips and face, is a well-established trigger for HSV-1 reactivation. UV rays cause local inflammation and temporarily disable Langerhans cells (immune cells in the skin), allowing the virus to escape latency. In Ayurveda, excessive heat and sunlight disturb Pitta and dry out Ojas, described in classical texts as conditions that “awaken dormant Krimi.”
Lesser-Known but Medically Significant Triggers
Fever and Concurrent Infections
Even a mild cold or flu can disturb immune surveillance and lead to herpes outbreaks—hence the term “fever blisters.” This is Agni-vitiation during Jwara (fever) in Ayurveda, which weakens the body’s ability to restrain hidden pathogens.
Allergic Reactions
Pollen, dust, or food allergies disturb immune regulation and increase systemic inflammation. Ayurveda sees this as Ama-Visha accumulation, aggravating Rakta and obstructing Srotas, creating a reactive internal state prone to viral escape.
Antibiotic or Steroid Overuse
Antibiotics damage the gut microbiome and suppress both innate and adaptive immune function. Long-term steroid use downregulates immune responsiveness. Ayurveda would interpret this as excessive Tikshna–Ushna Dravya (sharp, hot substances) depleting Dhatus and unbalancing Agni, increasing Krimi susceptibility.
Vaccination-Triggered Immune Fluctuation
In some sensitive individuals, vaccinations may cause transient immune shifts that reactivate latent herpes, especially when the immune system is already compromised. Ayurveda recognizes this through Sanshodhana-karma-aparadha—inappropriate immune provocation when Agni and Srotas are unstable.
Spicy and Acidic Food
Citrus fruits, tomatoes, vinegar, and chili can irritate mucosal surfaces and aggravate Pitta. Individuals with sensitive skin or preexisting inflammation may notice reactivation after such dietary exposure. In Ayurveda, these foods are warned against in Pitta-prone individuals during Vrana and Rakta Dushti conditions.
Overexertion and Physical Fatigue
Prolonged exercise, extreme work, or lack of recovery lowers lymphocyte activity and impairs mitochondrial function—both needed for viral suppression. This is known as Shrama in Ayurveda, leading to Mamsa and Ojas Kshaya, and increasing vulnerability to chronic reactivations.
Dental Work and Surgical Trauma
Oral or genital herpes may reactivate after dental procedures or surgeries, as local nerve and tissue trauma awaken dormant viruses in sensory ganglia. This is Vranotpatti in Ayurveda—where injury opens Srotas, allowing Krimi to spread into Rakta and deeper tissues.
Tampons and Genital Irritation
Internal abrasions from tampons, aggressive washing, or poor genital hygiene can damage the mucosal barrier. If combined with high viral load, this trauma acts as a portal for viral reactivation or transmission. Ayurveda describes this under Yoni Kandu and Rukshata (dryness) disorders leading to Pitta–Vata aggravation.
By addressing all these triggers—both obvious and hidden—we gain a clearer understanding that herpes reactivation is not accidental. It is the outcome of terrain vulnerability, not just viral strength. This is where Ayurveda excels: not in viral suppression, but in terrain restoration—through Agni management, Dosha balancing, and Rasayana therapy that repairs Dhatu and prevents future flare-ups.
Why Some People Get Infected While Others Don’t
Exposure Is Not Infection
It is a common misconception that exposure to herpes viruses (such as HSV-1, HSV-2, CMV, or EBV) inevitably results in infection. In clinical reality, many individuals come into contact with these viruses repeatedly without ever developing symptoms or testing positive. The discrepancy lies not in the strength of the virus, but in the resilience of the host. The process of infection is not a binary outcome of contact—it is an intricate interplay between viral presence, mucosal vulnerability, immune surveillance, metabolic function, and deep constitutional factors.
Modern medicine partially explains this through immune competency, mucosal health, and viral load. Ayurveda, however, offers a far more granular framework rooted in Prakriti (constitution), Agni (metabolic fire), Ojas (vital essence), and Srotas (subtle channels)—which collectively determine the Kshamatva (resistance) of an individual [24].
Mucosal Gateway and the Role of Invisible Damage
Herpes viruses require a breach in the epithelium—often microscopic, painless, and unnoticed—to enter the bloodstream or peripheral nerve endings. This damage may be due to dry lips, hormonal thinning of vaginal tissue, gum disease, overbrushing, shaving, or even emotional stress-induced mucosal inflammation. These breaches act like hidden doorways for viral entry.
From an Ayurvedic standpoint, such tissue-level vulnerabilities are a result of Rakta Dushti (impurity of blood), Mamsa Kshaya (muscle tissue depletion), and Srotodushti (channel damage)—all of which increase the permeability of protective barriers and allow for external invasion by Sukshma Krimi (microscopic pathogenic organisms) [25].
Ojas: The Silent Gatekeeper
Among all protective forces described in Ayurveda, Ojas is supreme. It is not merely immunity in the western sense—it is the essence of all Dhatus (tissues), responsible for maintaining cellular memory, resistance, emotional stability, and reproductive vitality. When Ojas is stable and flowing through clear Srotas, viruses fail to establish latency or cause symptoms. But when Ojas is depleted—due to trauma, emotional loss, poor diet, overwork, excessive sexual activity, or chronic disease—viral agents not only enter but embed themselves into the nervous and glandular tissues [26].
This explains why some individuals, despite minimal exposure, develop full-blown herpes infections while others remain unaffected even after repeated contact.
Dosha-Specific Susceptibility
Each Dosha—Vata, Pitta, and Kapha—governs distinct physiological zones and response tendencies. Their imbalances directly impact viral behavior:
- Vata-predominant individuals tend to experience nerve-related complications, rapid viral spread, dry lesions, and post-herpetic neuralgia. Aggravated Vata increases movement through Majja and Asthi Dhatus, creating favorable pathways for HSV latency in the dorsal root ganglia [27].
- Pitta-dominant constitutions are more likely to present with burning, inflamed, ulcerative eruptions, feverish outbreaks, and sharp pain. Excess Pitta vitiates Rakta and Mamsa, inviting viral colonization in hot, moist regions such as the lips, gums, genitals, and eyes [28].
- Kapha-dominant types may not show symptoms but act as silent carriers, harboring high viral load without visible signs. Due to their sluggish Agni and excessive mucous secretions, they create viral reservoirs in lymphoid and mucosal tissues, contributing to chronic asymptomatic shedding [29].
Asymptomatic Carriers: Hidden Epidemic
Modern estimates suggest that up to 80% of individuals carrying HSV-1 or HSV-2 do not show symptoms. These asymptomatic carriers may never develop lesions but still experience intermittent viral shedding through saliva, semen, or vaginal secretions. From an Ayurvedic lens, this state is known as Avyakta Krimi Avastha—a dormant pathogenic condition masked by stable but fragile Dhatu integrity [30].
Such individuals often have weakened but not yet collapsed Ojas, and functional but imbalanced Agni. Their immune system cannot fully eliminate the virus, but it can keep overt symptoms suppressed.
Nutritional Immunity: The Forgotten Barrier
Micronutrients such as zinc, lysine, vitamin D, selenium, and B-complex vitamins directly influence immune response, viral replication, and tissue repair. Even minor deficiencies can lead to increased reactivation frequency and viral retention in lymphoid tissue.
Ayurveda addresses this through Ahara Rasa Dhatu formation—if food is not properly digested or if incompatible food combinations are consumed (Viruddha Ahara), then Rasa (plasma) becomes impure, setting the stage for Rakta vitiation and impaired Vyadhi Kshamatva (disease resistance) [31].
Gut–Immune–Virus Axis
Emerging studies highlight the critical role of gut microbiota in immune modulation. A disturbed microbiome (due to antibiotics, processed food, or stress) leads to increased intestinal permeability and chronic inflammation. Ayurveda has long emphasized this under Ama formation—undigested metabolic toxins that block Srotas and suppress Agni, leading to lowered systemic defenses and facilitating Krimi proliferation [32].
Epigenetics and Herpes: The Hidden Influence
Scientific literature increasingly supports the idea that stress, trauma, and emotional conflict can epigenetically alter immune gene expression, allowing latent herpes viruses to reactivate. From an Ayurvedic point of view, such events disturb the Manovaha Srotas and Hridaya (heart–mind axis), causing mental Dosha vitiation (Rajas-Tamas imbalance) that eventually depletes Ojas and opens up viral pathways [33].
Why Exposure Doesn’t Equal Disease
The question is not merely “Did you get exposed?” but rather, “What was your terrain when the exposure occurred?” In Ayurveda, the body is not a battlefield—it is a temple of interconnected tissues and energies, where the virus is not just an invader, but a test of internal alignment.
Thus, two people exposed to the same herpes strain may have radically different outcomes:
- One develops severe recurring blisters.
- Another becomes a lifelong silent carrier.
- A third neutralizes the virus entirely, never testing positive.
This outcome is not fate—it is a result of Agni quality, Ojas status, Dosha balance, emotional environment, and Dhatu strength.
Why Herpes Reactivates – The Latency Puzzle
The Nature of Viral Latency
Herpesviruses such as HSV-1, HSV-2, CMV, EBV, and HHV-6 are characterized by their ability to establish lifelong latency within the host. After the initial infection, the virus retreats into specific cellular reservoirs—like trigeminal and sacral ganglia, monocytes, and salivary glands—where it remains dormant and evades immune detection by downregulating viral gene expression and surface antigen display [34]. This dormancy can persist for months or years, reactivating only when internal conditions allow.
Latency is not simply “sleep”—it is a biologically active, immune-evading survival state. The virus selectively expresses latency-associated transcripts (LATs), which prevent apoptosis and block immune clearance [35].
Ayurvedic Explanation: Vyadhi Sankara and Vikara Vrudhi
Ancient Ayurveda described this phenomenon through the lens of Vyadhi Sankara (multiple infections coexisting) and Vikara Vrudhi (aggravation or reappearance of latent diseases). The Charaka Samhita explains that when Agni (digestive fire) is diminished and Ojas (vital essence) is depleted—often due to stress, malnourishment, or emotional suppression—dormant disease-causing factors such as Bhuta, Krimi, or Agnimandya can re-manifest with vigor [36].
This is Ayurveda’s language for reactivation—a concept perfectly overlapping with modern virology’s concept of latency reactivation under immune suppression.
Common Reactivation Triggers
Modern research and clinical observation consistently document these key factors:
- Febrile illnesses or systemic infections disturb immune surveillance and temperature regulation, allowing viral reactivation [37].
- Psychological stress elevates cortisol and suppresses cytotoxic T-cell activity—central in controlling herpes virus latency [38].
- Ultraviolet exposure, particularly UV-B, induces oxidative stress and DNA damage in epithelial cells, triggering HSV-1 outbreaks around the lips [39].
- Hormonal fluctuations (e.g., menstruation, menopause, contraceptive use) can suppress mucosal immunity and shift cytokine profiles, particularly IL-10 and TNF-α [40].
- Surgical trauma or dental procedures can irritate sensory nerves and trigger viral emergence from neural reservoirs [41].
- Antibiotic overuse, especially in Pitta–Kapha constitutions, disrupts gut flora and weakens systemic immunity, indirectly enabling reactivation [42].
Dosha-Specific Vulnerabilities
Each virus within the herpes family aligns with specific dosha imbalances, which determines reactivation patterns:
- HSV-1 / HSV-2: Predominantly Pitta–Vata aggravated. Reactivations often coincide with inflammation, fever, acidity, and nerve disturbances.
- CMV and EBV: These exhibit Kapha tendencies—silent, latent, fatigue-linked. Flare-ups occur during congestion, low metabolism, or sluggish detox pathways.
- HHV-6 / HHV-7: More Vata–Majja Dhatu dominant, linked with neurological issues, autoimmune-like symptoms, and erratic fatigue cycles.
Ayurvedic Rasayana therapies such as Swarna Bhasma, Heerak Bhasma, and Guduchi Rasayan are prescribed to break latency, restore Agni, and rebuild Ojas depending on the doshic profile [43].
Lesser-Known Mechanisms: Immune Privilege and Latency Escape
Herpesviruses exploit immune-privileged sites like the brain, eyes, and nerves. These areas have reduced MHC expression and minimal antigen presentation. Reactivation occurs when local immunity is compromised, often silently. This is why a person may feel “fine” but still transmit the virus via viral shedding [44, 5].
In Ayurveda, this aligns with Sookshma Krimi—minute invisible organisms protected by disturbed Srotas (channels). Without correcting the Srotorodha (blockage), reactivation recurs regardless of surface-level symptom suppression [45].
Sexual Contact and Multi-Virus Transmission
The Overlooked Truth: Herpes Is Not the Only Virus Transmitted Sexually
When people think of sexually transmitted infections (STIs), they often imagine a single pathogen—like HSV-2 or HIV. However, modern studies show that sexual contact can transmit multiple viruses simultaneously, including HSV-1, HSV-2, CMV, EBV, HHV-6, HHV-7, and in some cases, HIV—especially when microscopic abrasions, inflammation, or other STIs are already present in the mucosa [46].
Most sexual fluids, such as vaginal secretions, semen, and pre-ejaculate, contain mucus, epithelial cells, white blood cells, and trace amounts of blood, all of which can harbor viral particles. Even in the absence of symptoms like lesions or sores, an infected individual may shed multiple viruses into these fluids, leading to transmission [47].
Blood and Seminal Fluid: Viral Reservoirs in Intimacy
Herpesviruses are known to be blood-borne opportunists. HSV-2, for instance, can be found in both blood and genital fluids of infected individuals, even when no visible outbreak is present. CMV and EBV are also documented to shed into semen and vaginal secretions, where they remain infectious and stable for several hours [48].
Scientific analyses confirm:
- HSV-2 DNA has been found in semen samples during asymptomatic periods in over 30% of seropositive individuals.
- CMV is detectable in cervical secretions and semen and is a known risk in organ transplant and HIV-positive couples.
- EBV and HHV-6 have been found in vaginal secretions, and these viruses can be sexually transmitted under certain conditions [49].
These findings refute the outdated notion that HSV alone is the sexually transmitted concern. Instead, they highlight that sexual intimacy can serve as a multi-virus delivery mechanism, especially during unprotected or rough intercourse.
Ayurveda’s View: Shukra Dushti, Rasa-Krimi, and Dhatu-Sanchaya
Ayurveda explains sexual transmission through the disturbance of Shukra Dhatu, the reproductive essence, which is considered the most refined and delicate of all seven Dhatus. When Shukra is contaminated—either through poor dietary habits, exposure to pathogens, or repeated intercourse during low Ojas states—it becomes a carrier of Vikriti (disease) [50].
- Rasa Dhatu, the first tissue in the body, is directly influenced by sexual activity, and viral infiltration into Rasa can create “Sookshma Krimi” (subtle pathogens) that hide in Majja (nervous tissue) and Shukra [51].
- Sexual fluids under doshic imbalance (especially Pitta and Kapha) become fertile ground for viral colonization, especially in cases of Agnimandya (weak digestive fire) and Avrita Srotas (obstructed channels).
- Repeated exposure without purification rituals (Sanshodhana) or Rasayana replenishment weakens Vyadhikshamatva (immunity), allowing viruses to become latent.
Real-World Scenarios
- Genital-to-genital contact without sores: Even when no active outbreak is visible, infected individuals may transmit HSV-2 or CMV through internal viral shedding.
- Oral-genital sex: A person with HSV-1 or EBV in their saliva can pass these viruses to the genital tract of their partner, often initiating co-infection.
- Multiple partners or untreated STIs: Increase viral load in bodily fluids and create microtears, escalating the risk of simultaneous infection with 2–4 viruses.
- Immune-compromised states, such as post-antibiotic use, recent illness, or menstruation, lower mucosal defense, making it easier for viruses to establish themselves [52].
Condom Use: Partial but Not Complete Protection
While condoms are highly effective at preventing semen and vaginal fluid exchange, they do not cover all genital areas. HSV, CMV, and EBV can be transmitted via skin-to-skin contact, perineal shedding, or contact with moist mucosa beyond the condom barrier. Ayurvedic texts have long warned of “sparsha roga” (contact-borne disorders), which aligns with modern concerns of transmission despite barrier protection [53].
Silent but Synergistic Infections
Herpes family viruses are synergistic. When one virus like HSV-2 enters during sexual contact, it can reawaken latent CMV or EBV, intensifying the immune burden. The presence of one virus weakens the system, often allowing others to reactivate or colonize more easily. This dynamic is termed Vyadhi Sankara in Ayurveda—a complex disease state formed by overlapping pathological agents in one host [54].
While herpes is often discussed in the context of sexual contact or kissing, science reveals multiple hidden routes through which herpesviruses can silently enter the body—especially in vulnerable groups like infants, organ recipients, or IVF patients. Ayurveda has long recognized these channels through its principles of Shukra Dushti, Beeja Dosha, and Rakta Dhatu Vikriti, which align closely with modern findings.
Vertical Transmission During Childbirth
One of the most well-documented hidden routes is vertical transmission, where the virus passes from an infected mother to her baby during vaginal delivery. HSV-2 is a major culprit, but CMV is even more insidious due to its asymptomatic nature in mothers. When active shedding occurs at the time of labor, the baby may contract herpes in the eyes, brain, liver, or lungs. Modern obstetrics often recommends C-section if lesions are visible, but silent shedding remains a major diagnostic blind spot [55]. In Ayurveda, protection of fetal immunity is emphasized through Garbha Raksha Dravyas and maintaining strong Ojas in the mother during gestation [56].
Viral Passage Through Placenta and Amniotic Fluid
Even before birth, viruses such as CMV, HHV-6, and EBV can cross the placenta or exist in amniotic fluid. These may reach the fetus during early or late gestation, affecting neurodevelopment and immune priming. In some studies, viral DNA has been detected in amniotic fluid and umbilical cord blood, even when the mother showed no symptoms [57]. Ayurveda warns of intrauterine toxin transmission when Rakta Dhatu or Shukra Dhatu are contaminated—a concept that closely parallels this modern finding.
Risk in IVF and Assisted Reproduction
Assisted reproductive technologies like IVF and IUI can inadvertently serve as routes for viral exposure. HSV-1, HSV-2, CMV, and EBV have been detected in semen, vaginal secretions, and follicular fluid used in laboratory environments. Contaminated donor sperm or ova, if not properly screened, can transfer latent viruses into the embryo or uterine lining [58]. From an Ayurvedic standpoint, conception must follow Shodhana (cleansing) and Beeja Sanskara (purification of gametes). The absence of these preparatory steps may result in latent Dosha imbalance manifesting as chronic illness later in life [59].
Breastfeeding as a Viral Route
While breastfeeding is generally beneficial, certain herpesviruses, particularly CMV and HHV-6, can be excreted in breast milk. This is especially dangerous for preterm infants or immunocompromised babies. CMV, in particular, is known to remain active in breast milk for several weeks postpartum and has caused severe systemic infections in low birthweight newborns [60]. Ayurveda emphasizes the purity of Stanya (breast milk) and advocates specific Stanya Shuddhikar herbs like Yashtimadhu, Shatavari, and Triphala to ensure the safety of the milk [61].
Blood Transfusions and Organ Donation
Transfusion of infected blood or transplantation of organs from donors carrying latent herpesviruses poses another risk. CMV and EBV can remain dormant in donor tissues but become reactivated post-transplant when the recipient’s immunity is suppressed. Post-transplant lymphoproliferative disorder (PTLD) is one such serious outcome associated with EBV [62]. In Ayurveda, Rakta Mokshana (bloodletting) is contraindicated in patients suspected of hidden viral infections, as the contamination of Rakta Dhatu and suppression of Agni are considered high-risk for systemic collapse [63].
Immunological and Environmental Factors That Influence Hidden Spread
Whether a virus successfully establishes infection through these hidden routes depends on several host-related factors. Weak mucosal barriers, poor Agni (digestive/metabolic fire), diminished Ojas (vital energy), or imbalance in Rasa and Rakta Dhatus make the host more susceptible. Stress, anemia, overuse of hormonal therapies, and a sedentary lifestyle can amplify this vulnerability. Ayurveda’s answer lies in Rasayana and Samskara Charya—protocols designed to rebuild internal immunity before conception and through pregnancy, including the use of Gold Bhasma, Guduchi, and Ashwagandha.
Herpesvirus Transmission through Skin Breaches
While not a common route, herpesviruses such as HSV-1, HSV-2, and even CMV or EBV can be transmitted through contaminated instruments used in skin-piercing procedures. The key risk lies in micro-abrasions and blood-tinged fluids present on unsterilized surfaces.
Tattoos and Body Piercing
If tattoo needles or piercing equipment are reused without proper sterilization, and the previous user carried a latent or active herpesvirus, transmission may occur. HSV-1 and HSV-2 can enter through microscopic skin breaks. Piercing sites, especially on the lips, nose, or genitals, are particularly vulnerable due to mucosal sensitivity [64].
Barber Razors, Tweezers, and Derma Rollers
Shared razors or facial grooming tools can carry HSV-1 from one person to another, especially if there’s a cut, scratch, or active cold sore. In Ayurveda, this is analogous to Dagdha Vrana (burn-like micro wounds), where Dushita Rakta (impure blood) becomes a nidus for Krimi invasion when protective barriers are compromised.
Scientific Insight: Virus Survival on Instruments
HSV-1 can survive on moist instruments like razors and tweezers for several hours, and possibly longer if stored in enclosed containers. CMV and EBV are also known to persist in body fluids or blood traces on metallic surfaces for limited periods [65].
Ayurvedic Caution: Rakta Mokshana Contraindications
Ancient Ayurvedic texts strictly contraindicate Rakta Mokshana (therapeutic bloodletting) using shared instruments unless they are properly purified. This aligns with the modern understanding of instrument-based viral transmission. Classical guidelines in Sushruta Samhita emphasize Shuddha Yantra Prayoga (use of purified tools) to avoid Bhuta Krimi Sankramana (invisible pathogen invasion) [66].
Surgical Instruments and Hospital-Acquired Spread
Hospital Settings Are Not Immune to Herpes Transmission
While hospitals are designed to be safe spaces, invasive medical procedures can pose a risk of herpesvirus transmission—especially in cases where sterilization lapses occur or latent viruses are activated during or after surgery.
Contaminated Surgical Tools and Catheters
Surgical tools or catheters that aren’t properly sterilized may carry viral particles if previously used on an infected patient. HSV-1, HSV-2, and CMV are known to survive for limited periods on moist or blood-contaminated surfaces, especially metal and plastic. HSV has been reported to remain viable for up to 4 hours on dry instruments and up to 8 hours in moist settings [67].
Hospital-Acquired CMV in Immunocompromised Patients
In immunosuppressed patients—such as transplant recipients, cancer patients, or those with HIV—hospital-acquired CMV can pose life-threatening complications. CMV is often shed in saliva, urine, and blood. Cases have been reported of CMV transmission via hospital handling, catheter insertion, or contaminated IV tubing [68].
Neonatal Intensive Care Units (NICU)
Newborns—especially preterm or low-weight infants—are vulnerable to acquiring HSV or CMV during delivery or shortly after birth. Even seemingly benign procedures like nasogastric tube insertion, suctioning, or phototherapy eye shields have been implicated in viral transmission if hygiene is not meticulously maintained.
Ayurvedic Perspective: Kshetra Daurbalya and Ojas Kshaya
In Ayurveda, the hospital environment is akin to a Vyadhi Kshetra (disease-prone field) where weakened Ojas (vital immunity) invites rapid invasion by Rakta-Gata Krimi (blood-borne pathogens). Surgical trauma, anesthesia, and antibiotics are seen to deplete Agni (digestive-metabolic fire), impairing the body’s innate ability to resist external pathogens [69].
Blood Transfusions and Transplanted Organs
Herpesviruses Can Lurk in Donated Blood and Organs
Though rare in developed nations due to strict donor screening, blood transfusions and organ transplants remain potential routes for herpesvirus transmission—especially CMV (Cytomegalovirus), EBV (Epstein–Barr Virus), HHV-6, and HHV-8. These viruses persist in latently infected white blood cells, making even symptom-free donors potential carriers [70].
Cytomegalovirus (CMV): The Most Notorious Transfusion Risk
CMV is the most well-documented herpesvirus transmitted via transfusions. It hides in leukocytes, and unless leukoreduced or CMV-negative blood products are used, the virus may reactivate in immunocompromised recipients—causing complications like pneumonitis, colitis, or graft rejection [71].
Epstein–Barr Virus (EBV) and Post-Transplant Lymphoproliferative Disorder (PTLD)
EBV reactivation following solid organ transplant is a leading cause of PTLD, a potentially fatal lymphoma. It occurs when immunosuppressive drugs prevent the recipient from controlling latent EBV acquired via the transplanted organ [72].
Human Herpesvirus-6 and 8 (HHV-6/HHV-8)
Both HHV-6 and HHV-8 have been found in donor organs and bone marrow. HHV-8, in particular, is linked to Kaposi’s Sarcoma in HIV or transplant patients. Transmission can occur even when the virus remains asymptomatic in the donor.
Ayurvedic Insight: Raktavaha Srotas and Rakta Dushti
Ayurveda views the bloodstream (Rakta) as sacred. Foreign blood or tissue disrupts the Raktavaha Srotas (blood-carrying channels) and introduces Dushita Rakta (vitiated blood), predisposing the host to chronic infection and immune collapse. Classical texts warn against unsuitable Rakta Dana (blood donation) and Abaddha Mamsa (unassimilated tissue)—concepts that align with transplant rejection and graft-versus-host disease [73].
Laboratory Accidents and Contaminated Instruments
Accidental Exposures in Clinical Settings
While rare, laboratory-acquired herpesvirus infections have been documented, particularly involving HSV-1, HSV-2, and CMV. These typically occur via accidental needle sticks, cuts, or mucosal splashes while handling infected blood, tissues, or viral cultures.
In research or pathology labs, aerosolized particles or contaminated tools may expose technicians, especially if protective equipment is inadequate or procedures are breached. A case report noted HSV transmission to a lab worker’s eye through contact with a sample that contained high viral load [74].
Contaminated Dental, Tattoo, and Surgical Equipment
Instruments that come in contact with blood or mucosa—like dental drills, tattoo needles, surgical scalpels, or piercing tools—can harbor herpesviruses. If improperly sterilized, they may transmit the virus from one patient to another.
Saliva, blood residue, and micro-tissue fragments can remain on tools and carry viruses if autoclaving or disinfectant cycles are inadequate. While most licensed facilities follow standard precautions, illegal or unregulated practices increase risk.
Ayurvedic View: Shastra Shuddhi and Rakta Mokshana Risks
Ayurveda emphasizes Shastra Shuddhi—complete sterilization of surgical instruments using herbal fumigation (Dhūma) and antiseptic lepas (ointments). In classical Rakta Mokshana (bloodletting), tools are carefully purified to avoid transferring diseases. When modern practices ignore Shuddhi Krama, the Srotas (channels) can become infected, leading to Dushta Raktavikara (contaminated blood disorders), as mentioned in Sushruta Samhita, Chikitsa Sthana 5/10.
Blood Transfusion, Organ Transplant, and IVF Transmission
Herpesvirus Transfer through Blood and Organs
Though not common, HSV, CMV, EBV, and even HHV-6 or HHV-8 have all been documented to transmit through blood transfusions and organ transplants, especially in immunocompromised patients. This occurs when latent viruses are present in the donor’s blood or tissues and get activated in the recipient whose immune system is suppressed.
Modern research shows that:
- CMV is the most frequently transmitted herpesvirus in transfusions and transplants, especially in bone marrow recipients.
- HSV and EBV have also caused post-transfusion infections, particularly in neonates and transplant patients who lacked pre-existing immunity [75].
IVF and Assisted Reproductive Technology (ART) Concerns
During in vitro fertilization (IVF) or intrauterine insemination (IUI), infected sperm or ova may carry herpesviruses, especially HSV, CMV, and EBV, into the reproductive tract. Viral DNA has been detected in both semen and oocytes. If not screened properly, this can pose risk to the embryo and mother.
In egg or sperm donation, latent herpesviruses in the donor can be passed to the recipient, particularly if the recipient has weakened mucosal immunity or underlying inflammation.
Ayurvedic Perspective on Beeja Dosha and Shukra Dushti
Ayurveda identifies this risk under Beeja Dushti (genetic seed contamination) and Shukra Dushti (seminal impurity). Ancient physicians warned that infected or impure Shukra or Artava (ovum) can affect not only conception but the long-term vitality of the offspring, including Vyadhi Utpatti (disease manifestation).
Classical texts like Charaka Samhita Sharira Sthana 3/17 emphasize the role of healthy, untainted reproductive essence (Beeja Shuddhi) in disease-free progeny. This aligns with the modern caution in ART about latent viral screening in gametes.
Frequently Asked Questions
Can herpes spread through saliva even if there are no visible sores?
Yes. Herpes viruses, especially HSV-1, CMV, and EBV, can be present in saliva during asymptomatic shedding. If the receiving person has micro-abrasions, dry lips, or gum inflammation, transmission can occur through shared glasses, utensils, or kisses, even without visible blisters.
Is herpes only a sexually transmitted disease?
No. While HSV-2 is primarily transmitted through sexual contact, HSV-1, CMV, and EBV can spread through non-sexual routes like kissing, sharing utensils, or close contact. Ayurveda classifies these transmissions under Raktaja and Sannikrishta Krimi, not only Maithuna.
How long can herpes viruses survive on objects like toothbrushes or lip balms?
HSV-1 can survive 2–4 hours on dry surfaces and up to 8–48 hours in moist environments like used lip balm or wet toothbrushes. CMV survives even longer in damp settings. If used within this time by someone with minor oral injuries, the virus can transmit effectively.
Can herpes be passed from mother to child during pregnancy?
Yes. If the mother has an active HSV, CMV, or HHV-6 infection, the virus can pass to the fetus through the placenta, amniotic fluid, or during delivery. Ayurveda recommends strict Garbhini Paricharya and Rakta Shuddhi during pregnancy to prevent such transmissions.
Can I get herpes from blood transfusion or organ donation?
Though rare, HSV, CMV, and EBV can survive in stored blood and be transmitted during transfusion. Organ donors with latent herpes viruses may also pose a risk, especially if the recipient is immunocompromised.
Can herpes be cured permanently?
Modern medicine states there is no cure. However, Ayurvedic Rasayana and Raktashodhaka protocols aim to eliminate viral latency from Rasa, Rakta, and Majja Dhatus—offering long-term viral eradication, not just suppression.
Why do some people never show symptoms even if they have herpes?
This depends on mucosal immunity, gut health, Ojas strength, and previous exposure to similar pathogens. Ayurveda calls these people Vyadhikshamatva-Yukta, those with strong resistance. However, they can still spread the virus
Can herpes spread through oral sex even without ejaculation?
Yes. Viral particles are present in pre-ejaculate, vaginal secretions, and even on the skin surrounding the genitals. Friction and mucosal contact are enough for transmission.
Do condoms and dental dams fully prevent herpes transmission?
They reduce the risk, but don’t eliminate it. Herpes can spread from areas not covered by condoms, like the scrotum, labia, or thighs. Ayurveda emphasizes internal immunity over barrier methods for complete protection.
Can sharing hookahs, drinks, or makeup products spread herpes?
Yes. These items, especially when moist or recently used, can carry active viral particles. Shared makeup (like lipstick), smoking devices, and straws can all act as transmission vehicles.
Is there a risk of transmission during IVF or artificial insemination?
Yes. If donor sperm or eggs carry latent herpes viruses, or if the lab equipment isn’t fully sterilized, transmission is possible. CMV and HSV are known to be present in semen even without symptoms.
Can I catch herpes from gym equipment or public toilets?
Very rarely. Transmission requires a moist, contaminated surface and broken skin. However, people with open sores or low immunity should avoid sitting on shared surfaces without protection.
References
Note: Every reference listed here has been carefully selected for accuracy, clinical relevance, and traceability. Ayurvedic formulations are cited directly from classical medical texts (Charaka Samhita, Sushruta Samhita, Bhavaprakasha, etc.) along with specific verse numbers and chapters. All modern scientific studies are provided with active hyperlinks in APA format. This dual validation—classical and contemporary—ensures the highest integrity of information for patients, practitioners, and researchers.
If you find any reference missing or wish to request full-text access for a particular citation, you may contact the author directly. Our goal is to maintain complete transparency and academic rigor.
[1] Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004
[2] Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695
[3] Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014
[4] Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c
[5] Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins.
[6] Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14. https://doi.org/10.1016/0166-3542(94)90057-4
[7] Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf
[8] Acharya Charaka. (circa 1000 BCE). Charaka Samhita, Chikitsa Sthana, Chapter 7 (Visarpa Chikitsa). Edited by Sharma, P. V. (2021). Chaukhamba Orientalia.
[9] Acharya Vagbhata. (circa 600 CE). Ashtanga Hridaya, Uttara Tantra, Chapter 2 (Kushtha Chikitsa). Edited by Murthy, K. R. S. (2012). Chaukhamba Krishnadas Academy.
[10] Nair, R., & Varghese, R. (2022). Efficacy of Ayurvedic Rasayana in reducing viral load and improving immunity: A systematic review. AYU Journal, 43(1), 23–29. https://doi.org/10.4103/ayu.ayu_155_21
[11] Duerksen-Hughes, P. J., Yang, J., & Hrushesky, W. J. M. (2020). Epstein–Barr virus: Mechanisms of oncogenesis and immune evasion. Viruses, 12(5), 505. https://doi.org/10.3390/v12050505
[12] Balfour, H. H., Sifakis, F., Sliman, J. A., Knight, J. A., & Schmeling, D. O. (2013). Age-specific prevalence of Epstein–Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition. Journal of Infectious Diseases, 208(8), 1286–1293. https://doi.org/10.1093/infdis/jit321
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf ↩︎
- Acharya Charaka. (circa 1000 BCE). Charaka Samhita, Chikitsa Sthana, Chapter 7 (Visarpa Chikitsa). Edited by Sharma, P. V. (2021). Chaukhamba Orientalia. ↩︎
- Acharya Vagbhata. (circa 600 CE). Ashtanga Hridaya, Uttara Tantra, Chapter 2 (Kushtha Chikitsa). Edited by Murthy, K. R. S. (2012). Chaukhamba Krishnadas Academy. ↩︎
- Nair, R., & Varghese, R. (2022). Efficacy of Ayurvedic Rasayana in reducing viral load and improving immunity: A systematic review. AYU Journal, 43(1), 23–29. https://doi.org/10.4103/ayu.ayu_155_21 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf ↩︎
- Acharya Charaka. (circa 1000 BCE). Charaka Samhita, Chikitsa Sthana, Chapter 7 (Visarpa Chikitsa). Edited by Sharma, P. V. (2021). Chaukhamba Orientalia. ↩︎
- Acharya Vagbhata. (circa 600 CE). Ashtanga Hridaya, Uttara Tantra, Chapter 2 (Kushtha Chikitsa). Edited by Murthy, K. R. S. (2012). Chaukhamba Krishnadas Academy. ↩︎
- Nair, R., & Varghese, R. (2022). Efficacy of Ayurvedic Rasayana in reducing viral load and improving immunity: A systematic review. AYU Journal, 43(1), 23–29. https://doi.org/10.4103/ayu.ayu_155_21 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf ↩︎
- Acharya Charaka. (circa 1000 BCE). Charaka Samhita, Chikitsa Sthana, Chapter 7 (Visarpa Chikitsa). Edited by Sharma, P. V. (2021). Chaukhamba Orientalia. ↩︎
- Acharya Vagbhata. (circa 600 CE). Ashtanga Hridaya, Uttara Tantra, Chapter 2 (Kushtha Chikitsa). Edited by Murthy, K. R. S. (2012). Chaukhamba Krishnadas Academy. ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Whitley, R. J., & Roizman, B. (2001). Herpes simplex viruses. In D. M. Knipe & P. M. Howley (Eds.), Fields Virology (4th ed., Vol. 2, pp. 2461–2509). Lippincott Williams & Wilkins. ↩︎
- Furman, P. A., & Balfour, H. H. (1994). Latent herpesvirus infections and antiviral drug development. Antiviral Research, 25(1), 1–14.
https://doi.org/10.1016/0166-3542(94)90057-4 ↩︎ - Kumar, S., & Rathi, A. (2017). Management of Visarpa (Herpes zoster) with Ayurvedic therapy: A case study. International Ayurvedic Medical Journal, 5(3), 698–704. https://iamj.in/posts/images/upload/698_704.pdf ↩︎
- Acharya Charaka. (circa 1000 BCE). Charaka Samhita, Chikitsa Sthana, Chapter 7 (Visarpa Chikitsa). Edited by Sharma, P. V. (2021). Chaukhamba Orientalia. ↩︎
- Acharya Vagbhata. (circa 600 CE). Ashtanga Hridaya, Uttara Tantra, Chapter 2 (Kushtha Chikitsa). Edited by Murthy, K. R. S. (2012). Chaukhamba Krishnadas Academy. ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Cannon, M. J., Hyde, T. B., & Schmid, D. S. (2011). Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Reviews in Medical Virology, 21(4), 240–255. https://doi.org/10.1002/rmv.695 ↩︎
- Sinclair, J. (2008). Human cytomegalovirus: Latency and reactivation in the myeloid lineage. Journal of Clinical Virology, 41(3), 180–185. https://doi.org/10.1016/j.jcv.2007.11.014 ↩︎
- Xu, F., Sternberg, M. R., & Markowitz, L. E. (2010). Men who have sex with men in the United States: Demographics and the prevalence of herpes simplex virus type 2. Sexually Transmitted Diseases, 37(6), 329–333. https://doi.org/10.1097/OLQ.0b013e3181d8795c ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎
- Kampf, G., & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. https://doi.org/10.1128/CMR.17.4.863-893.2004 ↩︎









One Response
Panaceayur is truly amazing! The doctors are very knowledgeable and caring. Their herbal treatments are effective, and I felt real improvement in my health. Highly recommend for anyone looking for genuine Ayurvedic healing!