- Lymphoma
- Classification of Lymphoma
- Pathophysiology of Lymphoma
- Risk Factors and Etiology
- Clinical Presentation of Lymphoma
- Diagnostic Approach
- Staging and Prognostic Scoring
- Conventional Treatment Modalities
- Side Effects and Long-Term Complications
- Ayurvedic Therapeutic Approach for Lymphoma
- Appeal
- Prognosis and Survivorship
- Case Study
- References and Further Reading
Lymphoma
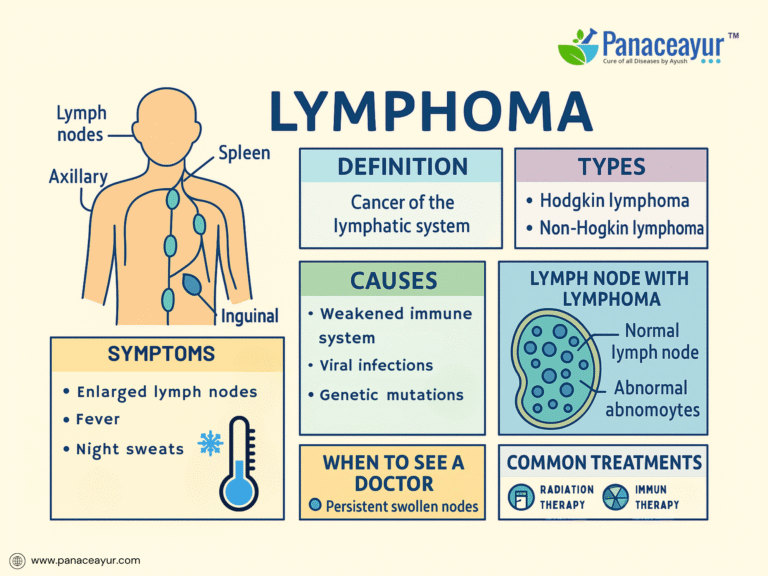
Lymphoma is a type of cancer that originates in the lymphatic system, a critical component of the body’s immune defense network responsible for filtering pathogens and maintaining fluid balance. Unlike other cancers that begin in solid organs, lymphoma arises from lymphocytes, the white blood cells that circulate through lymph nodes, the spleen, and other lymphatic tissues. Broadly classified into Hodgkin lymphoma (HL) and Non-Hodgkin lymphoma (NHL), this disease represents a diverse group of malignancies with varying clinical behaviors, prognoses, and treatment responses. Globally, lymphoma accounts for approximately 3% of all cancers, with an estimated 544,000 new cases and 260,000 deaths annually according to recent data from the Global Cancer Observatory (GLOBOCAN, 2020). While Hodgkin lymphoma has become highly curable with modern therapies, many forms of Non-Hodgkin lymphoma remain challenging due to their heterogeneity and risk of relapse. Early detection plays a pivotal role in improving outcomes, yet symptoms such as painless lymph node swelling, fatigue, and night sweats are often overlooked or misattributed to benign conditions. As advances in molecular diagnostics and targeted therapies continue to evolve, understanding the basic foundations of lymphoma is crucial for both patients and healthcare providers navigating the complexities of this disease.
Classification of Lymphoma
Lymphoma is broadly classified into Hodgkin lymphoma (HL) and Non-Hodgkin lymphoma (NHL), a distinction based on unique pathological and clinical features. Hodgkin lymphoma, accounting for approximately 10% of all lymphomas, is characterized by the presence of Reed-Sternberg cells—large, abnormal B lymphocytes identifiable under microscopy. Hodgkin lymphoma is further subclassified into types such as nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted, each with distinct histological patterns and prognostic implications. In contrast, Non-Hodgkin lymphoma encompasses a highly heterogeneous group of malignancies arising from either B lymphocytes, T lymphocytes, or natural killer (NK) cells. The World Health Organization (WHO) classification system organizes NHL into multiple subtypes based on immunophenotype, genetic markers, and morphology, with diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma being the most prevalent. Additional rare but clinically significant variants include mantle cell lymphoma, Burkitt lymphoma, and peripheral T-cell lymphoma. The behavior of these lymphomas varies widely, ranging from indolent, slow-growing forms to aggressive subtypes requiring immediate treatment. Understanding these classifications is crucial not only for accurate diagnosis but also for guiding treatment decisions, predicting prognosis, and selecting targeted therapies based on molecular characteristics. Clinicians must also be vigilant for exceptional cases such as double-hit lymphomas or transformed lymphomas, where overlapping features or genetic alterations may complicate categorization and necessitate more intensive therapeutic approaches.
Pathophysiology of Lymphoma
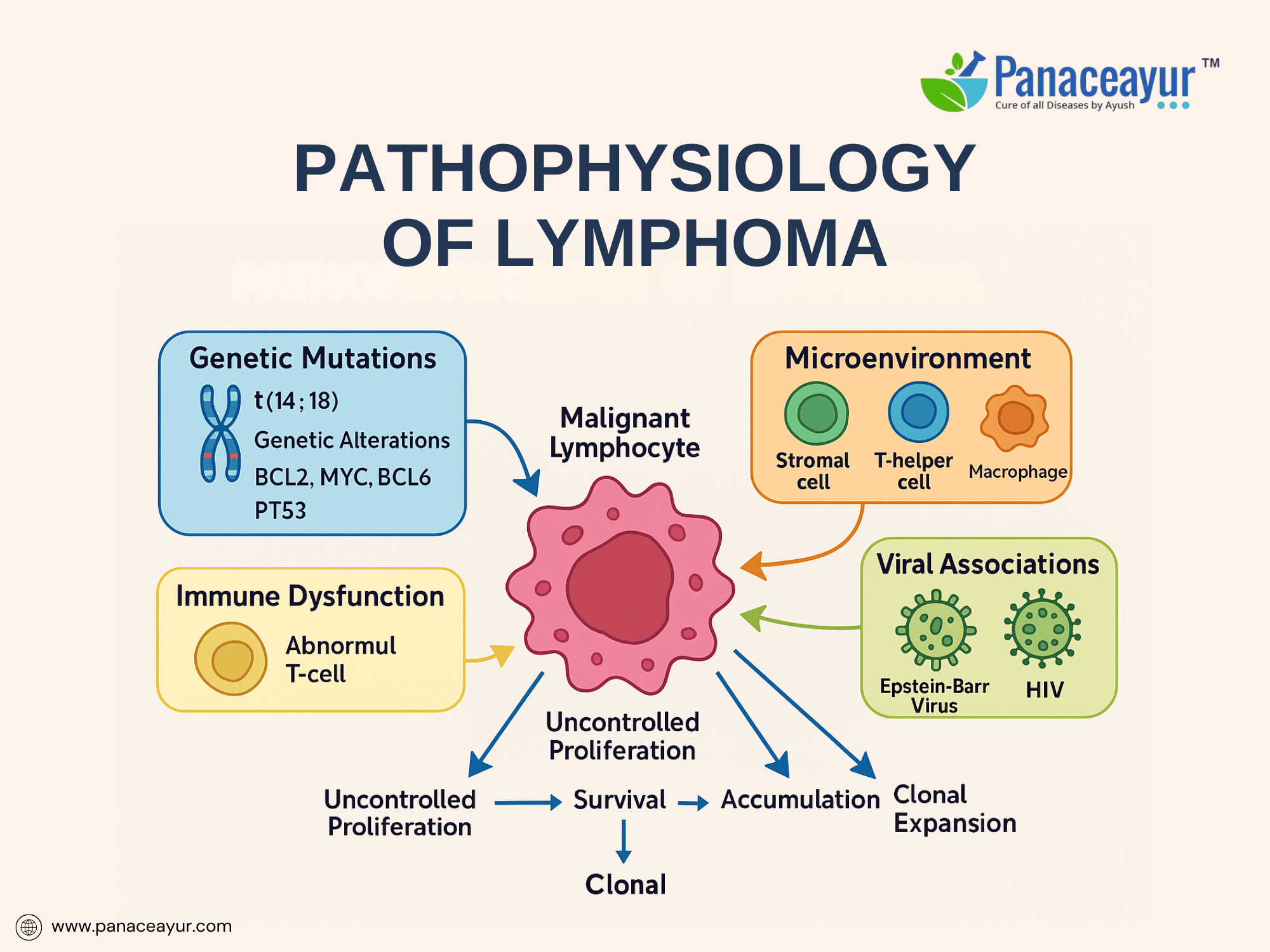
The pathophysiology of lymphoma is a multifactorial process involving a complex interplay of genetic mutations, epigenetic dysregulation, microenvironmental influences, and immune escape mechanisms that collectively disrupt normal lymphocyte development and homeostasis. At its core, lymphoma arises from the malignant transformation of B cells, T cells, or natural killer (NK) cells at various stages of differentiation within the lymphoid lineage. This transformation leads to the unchecked clonal proliferation of a population of abnormal lymphoid cells capable of evading normal apoptotic pathways and immune surveillance.
A central feature in many lymphomas is the occurrence of chromosomal translocations that juxtapose oncogenes next to immunoglobulin or T-cell receptor gene enhancers, resulting in their dysregulated expression. For instance, the hallmark translocation t(14;18)(q32;q21) places the anti-apoptotic gene BCL2 under the control of the immunoglobulin heavy-chain promoter in follicular lymphoma, leading to overexpression of BCL2 protein and inhibition of mitochondrial apoptosis pathways. Similarly, t(8;14)(q24;q32) translocation involving the MYC oncogene characterizes Burkitt lymphoma, resulting in constitutive MYC overexpression and driving unchecked cellular proliferation through enhanced transcription of genes involved in cell cycle progression and ribosomal biogenesis.
Beyond translocations, additional mutational events further contribute to lymphoma pathogenesis. Mutations in BCL6, a transcriptional repressor critical for germinal center formation, lead to sustained germinal center B-cell phenotypes and block differentiation into plasma cells in diffuse large B-cell lymphoma (DLBCL). Alterations in tumor suppressor genes such as TP53 compromise DNA damage response pathways, facilitating genomic instability and accumulation of additional oncogenic mutations. Emerging evidence has also implicated mutations in epigenetic regulators such as EZH2, CREBBP, and KMT2D, highlighting the role of aberrant chromatin remodeling and histone modification in sustaining the malignant phenotype.
An equally important dimension in lymphoma biology is the influence of the tumor microenvironment. Malignant lymphocytes exploit signals from surrounding stromal cells, follicular dendritic cells, macrophages, and T-helper cells to create a permissive niche that promotes survival, proliferation, and immune evasion. Cytokines such as interleukin-6 (IL-6), BAFF, and CXCL12 facilitate paracrine support of tumor cells, while interactions with PD-1/PD-L1 immune checkpoint pathways dampen cytotoxic T-cell responses, enabling immune escape. This microenvironmental dependency is especially evident in classical Hodgkin lymphoma, where the malignant Reed-Sternberg cells constitute only a small fraction of the tumor mass amidst an extensive inflammatory milieu recruited and co-opted to sustain tumor growth.
Viral oncogenesis represents another critical pathogenetic mechanism in certain lymphoma subtypes. Epstein-Barr virus (EBV), a gammaherpesvirus with tropism for B cells, contributes to lymphomagenesis through the expression of latent viral proteins such as LMP1 and EBNA1, which mimic CD40 signaling and interfere with apoptosis. EBV-positive lymphomas are particularly prevalent in immunocompromised hosts, such as individuals with HIV/AIDS or those undergoing immunosuppressive therapy post-transplant, reflecting the importance of intact immune surveillance in preventing virally mediated lymphoproliferation. Other oncogenic viruses, including HTLV-1 (in adult T-cell leukemia/lymphoma) and HHV-8 (in primary effusion lymphoma), underscore the role of viral antigens in driving lymphoid neoplasia through chronic antigenic stimulation, genomic instability, and inflammatory cytokine dysregulation.
The progressive accumulation of malignant lymphocytes within lymphoid tissues leads to architectural effacement, loss of nodal compartmentalization, and eventual infiltration into extranodal sites such as the gastrointestinal tract, central nervous system, skin, and bone marrow. This disrupts normal immune function, predisposes to opportunistic infections, and causes direct mass effect symptoms. Moreover, lymphoma cells can disseminate through lymphatic and hematogenous routes, contributing to widespread disease at diagnosis in many aggressive subtypes.
Importantly, the heterogeneity of molecular alterations across different lymphoma subtypes explains their divergent clinical behaviors, ranging from indolent lymphomas that remain asymptomatic for years to highly aggressive forms requiring urgent treatment. This molecular complexity provides a rationale for targeted therapies, such as Bruton’s tyrosine kinase (BTK) inhibitors, PI3K inhibitors, BCL2 antagonists, and immune checkpoint inhibitors, which exploit specific vulnerabilities within lymphoma cells. Understanding the molecular pathogenesis of lymphoma thus not only informs diagnostic and prognostic stratification but also paves the way for precision medicine approaches aimed at improving therapeutic outcomes.
Risk Factors and Etiology
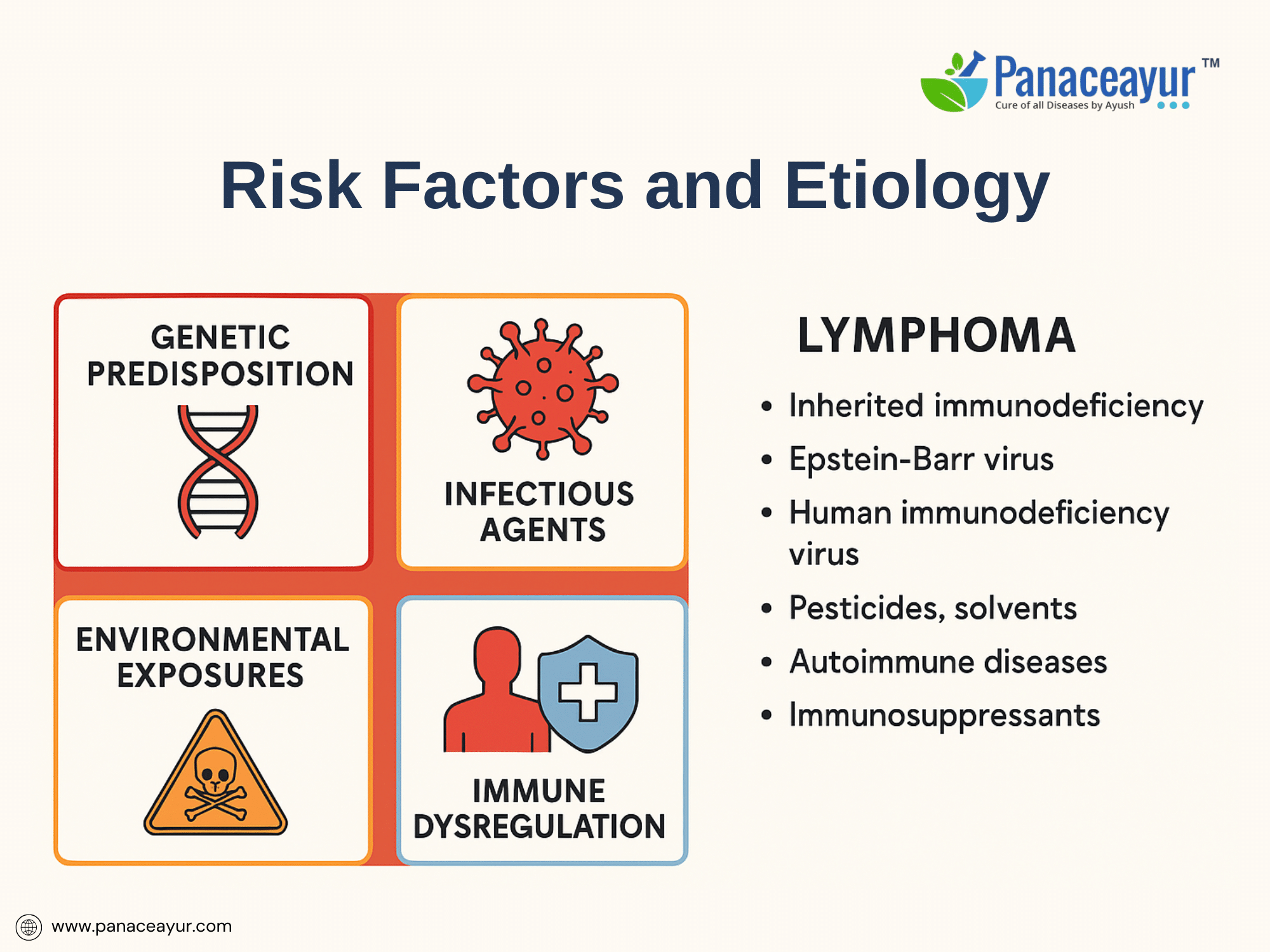
The etiology of lymphoma is multifaceted, involving a combination of genetic predisposition, environmental exposures, infectious agents, and immune dysregulation. While the exact cause of most lymphomas remains elusive, several well-established risk factors have been identified through epidemiological and molecular studies. Inherited genetic susceptibility plays a modest role, with familial clustering observed in certain cases, suggesting polygenic contributions or shared environmental triggers. Specific primary immunodeficiency syndromes, such as Wiskott-Aldrich syndrome and ataxia-telangiectasia, predispose individuals to lymphoid malignancies due to impaired DNA repair and chronic antigenic stimulation.
Exposure to oncogenic viruses is a significant etiological factor in select lymphoma subtypes. Epstein-Barr virus (EBV) is implicated in endemic Burkitt lymphoma, certain subtypes of Hodgkin lymphoma, and post-transplant lymphoproliferative disorders (PTLD), while human T-cell lymphotropic virus type 1 (HTLV-1) is causally linked to adult T-cell leukemia/lymphoma. Chronic infection with hepatitis C virus (HCV) has been associated with marginal zone lymphomas, likely mediated by antigen-driven B-cell proliferation. Additionally, Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) is involved in primary effusion lymphoma, particularly in immunosuppressed patients.
Environmental exposures contribute to lymphomagenesis, with studies identifying increased risk among individuals exposed to pesticides, herbicides, organic solvents, and certain industrial chemicals, particularly in agricultural and manufacturing settings. Ionizing radiation and prior chemotherapy also elevate lymphoma risk by inducing mutagenic DNA damage. Occupational exposure to benzene, for example, has been correlated with higher incidence of Non-Hodgkin lymphoma in population-based studies.
A critical factor in lymphoma development is immune dysregulation, either acquired or iatrogenic. Individuals with HIV/AIDS exhibit a dramatically increased risk of high-grade lymphomas, including primary central nervous system lymphoma and immunoblastic lymphoma, largely due to chronic immune activation and diminished immunosurveillance. Similarly, patients receiving long-term immunosuppressive therapy post-organ transplantation are susceptible to PTLD, reflecting the delicate balance between immune competence and oncogenic potential.
Interestingly, certain autoimmune diseases, such as Sjögren’s syndrome, rheumatoid arthritis, and celiac disease, are linked to an elevated risk of specific lymphoma subtypes, especially mucosa-associated lymphoid tissue (MALT) lymphoma, likely due to chronic antigenic stimulation leading to clonal B-cell expansion. Furthermore, Helicobacter pylori infection has been causally associated with gastric MALT lymphoma, and eradication of the bacterium can induce lymphoma regression in early stages.
The interplay between genetic lesions, chronic inflammation, antigenic stimulation, and impaired immune control underscores the heterogeneity of lymphoma pathogenesis. This complexity necessitates a personalized approach to risk assessment and highlights potential avenues for prevention, such as minimizing occupational exposures and treating underlying infectious or autoimmune triggers.
Clinical Presentation of Lymphoma
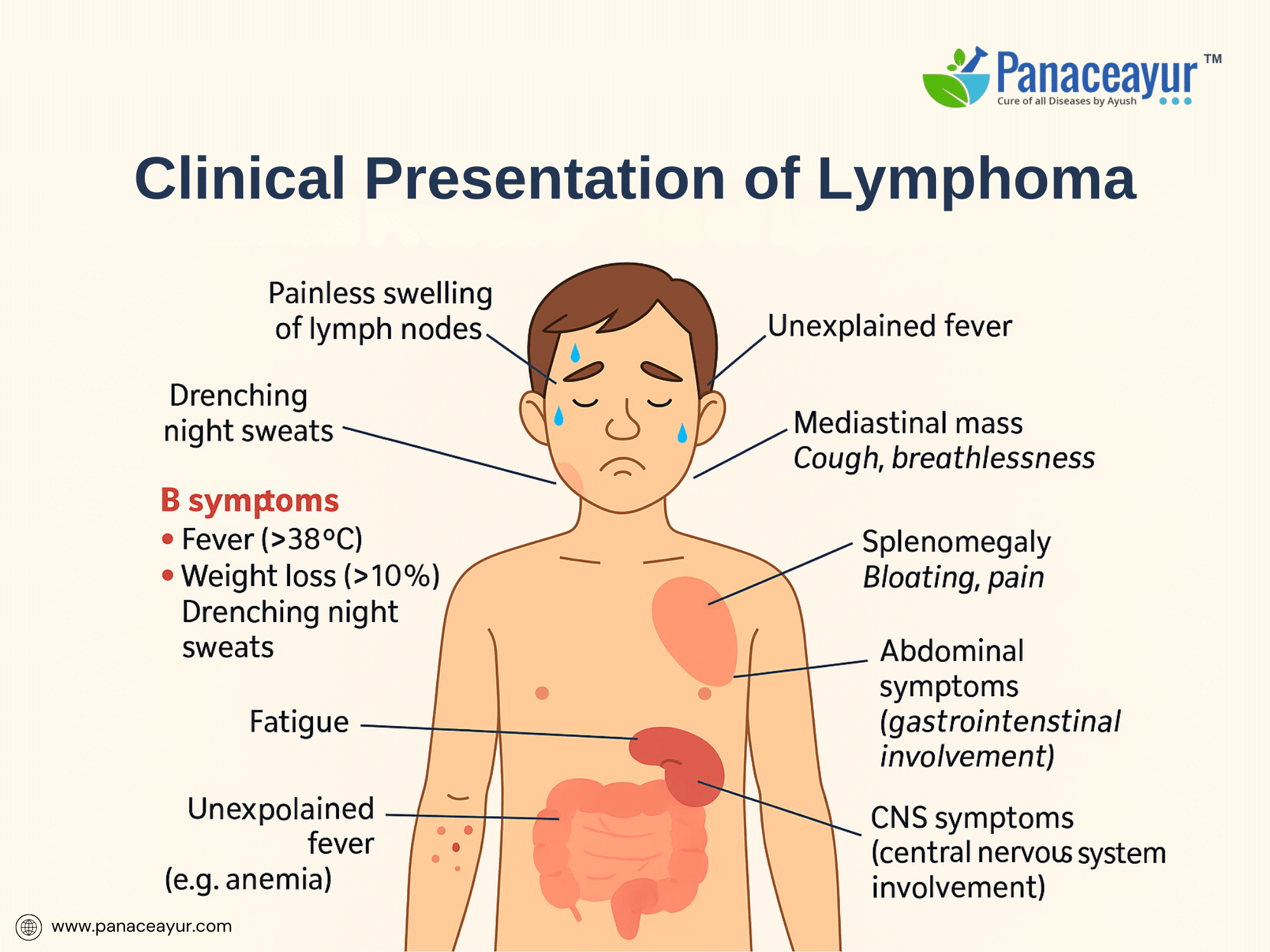
The clinical presentation of lymphoma is remarkably variable, depending on the specific subtype, disease stage, and the anatomical sites involved. The most common and often earliest manifestation is painless lymphadenopathy, typically affecting the cervical, axillary, or inguinal lymph nodes. Patients may notice firm, rubbery lymph nodes that progressively enlarge over weeks or months without tenderness or inflammation. In Hodgkin lymphoma, a characteristic pattern of contiguous nodal spread is observed, whereas Non-Hodgkin lymphoma may exhibit more discontinuous or extranodal involvement at diagnosis.
A hallmark of aggressive lymphomas, particularly Hodgkin lymphoma, is the presence of “B-symptoms”—a triad of unexplained fever exceeding 38°C, drenching night sweats, and significant (>10%) unintentional weight loss over six months. The presence of B-symptoms is associated with a higher tumor burden, systemic inflammatory response, and adverse prognosis, warranting their inclusion in staging and risk stratification models. Constitutional symptoms such as profound fatigue, anorexia, and malaise may accompany systemic disease.
Specific symptomatology also reflects the anatomical sites of lymphomatous infiltration. Mediastinal masses, commonly seen in nodular sclerosis Hodgkin lymphoma and primary mediastinal B-cell lymphoma, may cause cough, chest discomfort, dyspnea, or superior vena cava syndrome. Abdominal lymphadenopathy or splenomegaly may present as bloating, early satiety, or vague abdominal pain, while gastrointestinal tract involvement—more typical of certain T-cell and mucosa-associated lymphoid tissue (MALT) lymphomas—can lead to bleeding, obstruction, or malabsorption. Bone marrow infiltration may manifest as cytopenias, fatigue from anemia, increased infections due to neutropenia, or bleeding tendencies linked to thrombocytopenia.
Extranodal presentations are more frequent in Non-Hodgkin lymphoma, involving diverse sites such as the skin (cutaneous T-cell lymphoma, mycosis fungoides), central nervous system (primary CNS lymphoma), testes, thyroid, orbits, or bone. Such presentations can mimic other inflammatory, infectious, or neoplastic conditions, often delaying diagnosis. Primary CNS lymphoma, for example, may present with focal neurological deficits, altered mental status, or seizures, necessitating high clinical suspicion in at-risk populations such as HIV-positive patients.
Unusual or rare manifestations include paraneoplastic syndromes such as peripheral neuropathy, vasculitis, or autoimmune hemolytic anemia, reflecting immune dysregulation triggered by the malignant clone. Occasionally, lymphoma is incidentally discovered during imaging for unrelated complaints or routine medical evaluations.
Ultimately, recognizing the spectrum of presenting symptoms and correlating them with clinical context is vital for early detection. Misattribution of symptoms to benign causes or infections remains a challenge, especially in young, otherwise healthy individuals presenting with isolated lymphadenopathy or non-specific systemic complaints. A high index of suspicion combined with timely histopathological evaluation remains the cornerstone of accurate diagnosis.
Diagnostic Approach
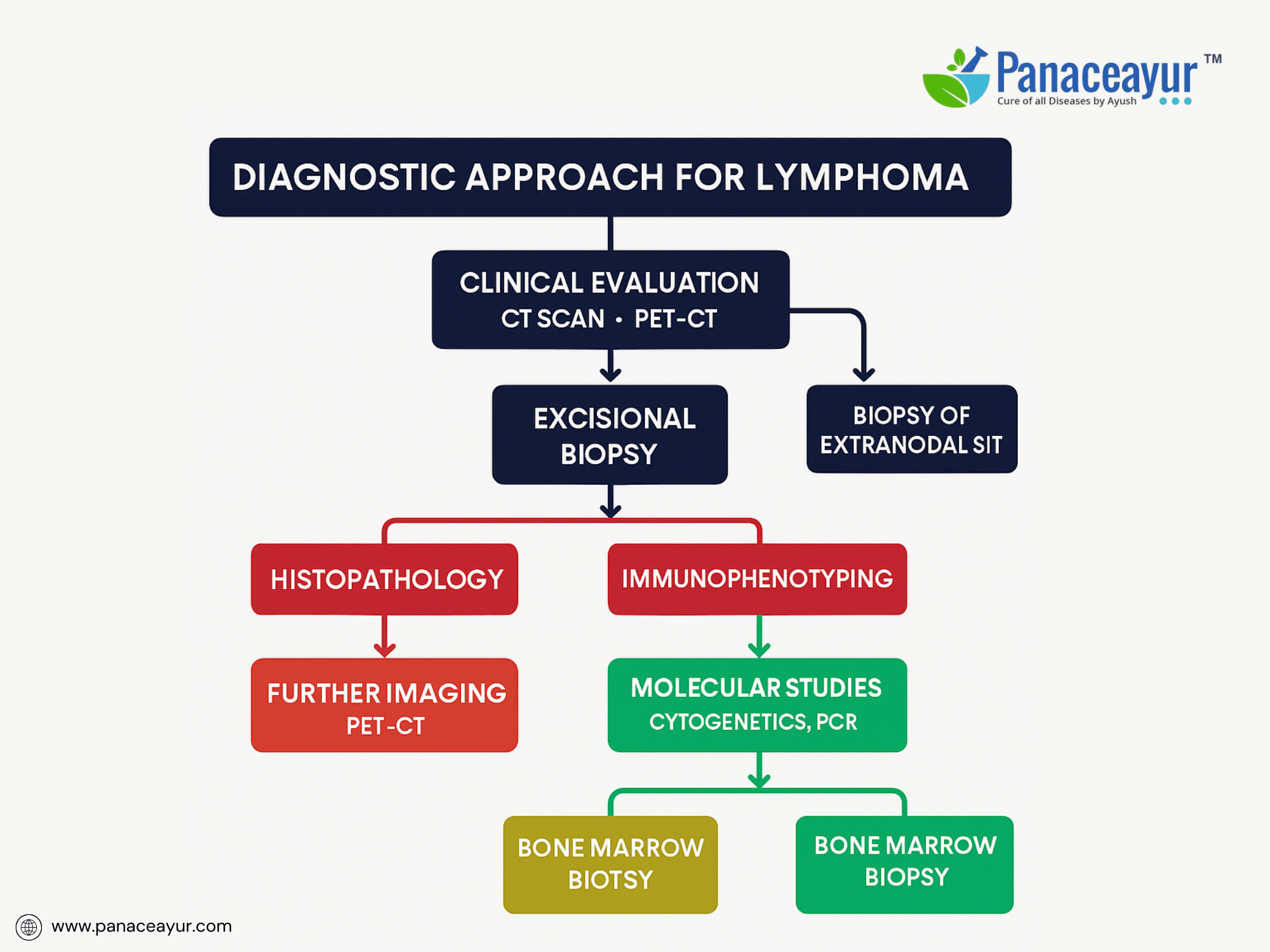
The diagnosis of lymphoma is a multi-tiered, interdisciplinary process requiring integration of clinical, morphological, immunophenotypic, cytogenetic, and molecular data to establish an accurate diagnosis, subtype classification, and staging. This comprehensive diagnostic framework is essential given the biological heterogeneity and therapeutic implications across more than 80 recognized lymphoma subtypes in the current World Health Organization (WHO) classification of Hematopoietic and Lymphoid Tissues.
At the foundation of lymphoma diagnosis is tissue histopathology, with excisional lymph node biopsy regarded as the gold standard due to its ability to preserve nodal architecture, allowing for the evaluation of follicular and paracortical zones, fibrosis, and sinusoidal involvement. Fine-needle aspiration (FNA) and core needle biopsy (CNB) are generally insufficient in isolation for definitive diagnosis except in inaccessible or high-risk anatomical sites, where CNB may be adjunctively used if immunohistochemistry (IHC) and flow cytometry are concurrently performed.
Microscopically, histopathological evaluation differentiates reactive hyperplasia from neoplastic proliferation, identifying hallmark features such as Reed-Sternberg cells in Hodgkin lymphoma, or monomorphic, atypical lymphoid infiltrates in Non-Hodgkin lymphoma. Subtyping requires assessment of cytomorphology (cell size, nuclear contours, chromatin pattern, mitotic activity) and tissue architecture (diffuse versus follicular growth patterns), which guide subsequent ancillary testing.
Immunohistochemistry (IHC) is pivotal in delineating lymphoid lineage and maturation stage, employing a battery of markers including CD45 (leukocyte common antigen), CD20 (B-cell marker), CD3 (T-cell marker), CD5, CD10, BCL2, BCL6, Ki-67 proliferation index, CD30, CD15, MUM1, Cyclin D1, ALK-1, among others. IHC also assists in detecting aberrant co-expression patterns, crucial in identifying composite or transformed lymphomas. For instance, CD5 positivity in mantle cell lymphoma (MCL) contrasts with CD5 negativity in follicular lymphoma, aiding differential diagnosis.
Flow cytometry immunophenotyping plays a critical adjunctive role, particularly in leukemic-phase lymphomas, small B-cell neoplasms, and T-cell lymphoproliferative disorders. Multicolor panels detect clonal populations via light chain restriction (kappa/lambda ratio) or aberrant antigen expression, facilitating distinction between reactive and neoplastic lymphoid expansions.
Cytogenetic and molecular genetic analyses are integral to lymphoma workup, contributing diagnostic, prognostic, and therapeutic insights. Fluorescence in situ hybridization (FISH) probes identify characteristic translocations such as t(14;18)(q32;q21) involving BCL2 in follicular lymphoma, t(11;14)(q13;q32) involving CCND1 (Cyclin D1) in mantle cell lymphoma, and MYC rearrangements (t(8;14)) in Burkitt lymphoma. Simultaneous detection of MYC and BCL2 and/or BCL6 rearrangements defines “double-hit” or “triple-hit” lymphomas, associated with highly aggressive clinical courses and distinct therapeutic protocols.
Molecular methods such as polymerase chain reaction (PCR) detect clonal immunoglobulin heavy-chain (IGH) or T-cell receptor (TCR) gene rearrangements, especially useful in cases with small or fragmented tissue samples, or ambiguous histology. Next-generation sequencing (NGS) panels are increasingly employed to identify somatic mutations (e.g., EZH2, MYD88, TP53, CARD11) that inform prognosis, targeted therapies, and clinical trial eligibility, moving lymphoma diagnostics toward a genomically informed precision medicine paradigm.
Imaging modalities are indispensable for disease localization, staging, and treatment response evaluation. 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT) is the imaging modality of choice in staging Hodgkin lymphoma and most aggressive Non-Hodgkin lymphomas, providing metabolic and anatomical information. PET/CT refines the Ann Arbor staging, differentiates active disease from fibrosis post-treatment, and guides biopsy of metabolically active sites. Contrast-enhanced CT remains an alternative in indolent lymphomas or where PET/CT is unavailable.
Bone marrow aspiration and trephine biopsy are routinely performed in staging of many Non-Hodgkin lymphomas to detect marrow involvement, influencing stage assignment and prognostication. Immunohistochemical staining for CD20, CD79a, or PAX5 enhances detection of subtle marrow infiltration.
Laboratory investigations provide important baseline and prognostic data. Elevated lactate dehydrogenase (LDH) reflects tumor burden and correlates with poor prognosis, incorporated into prognostic indices like the International Prognostic Index (IPI) or Hasenclever Index. Beta-2 microglobulin, a marker of tumor cell turnover, further refines risk stratification. HIV and hepatitis serologies are mandatory in high-risk populations, while uric acid assessment anticipates tumor lysis syndrome risk in bulky or high-grade disease.
Cerebrospinal fluid (CSF) analysis, including cytology and flow cytometry, is indicated in lymphomas with CNS tropism, such as primary CNS lymphoma, Burkitt lymphoma, or T-cell lymphoblastic lymphoma. Similarly, site-specific biopsies (GI tract, testis, thyroid, skin, CNS) may be required in extranodal presentations.
Emerging techniques, including liquid biopsy approaches (circulating tumor DNA, microRNA profiling), are under investigation for non-invasive disease monitoring, minimal residual disease detection, and dynamic assessment of tumor evolution.
Diagnostic workup must be contextualized within patient-specific factors, including performance status, comorbidities, reproductive planning, and cardiopulmonary reserve in anticipation of anthracycline-based chemotherapy or radiotherapy. Integration of pathologic, molecular, and imaging data mandates a multidisciplinary tumor board discussion to ensure diagnostic accuracy and optimal treatment planning.
Staging and Prognostic Scoring
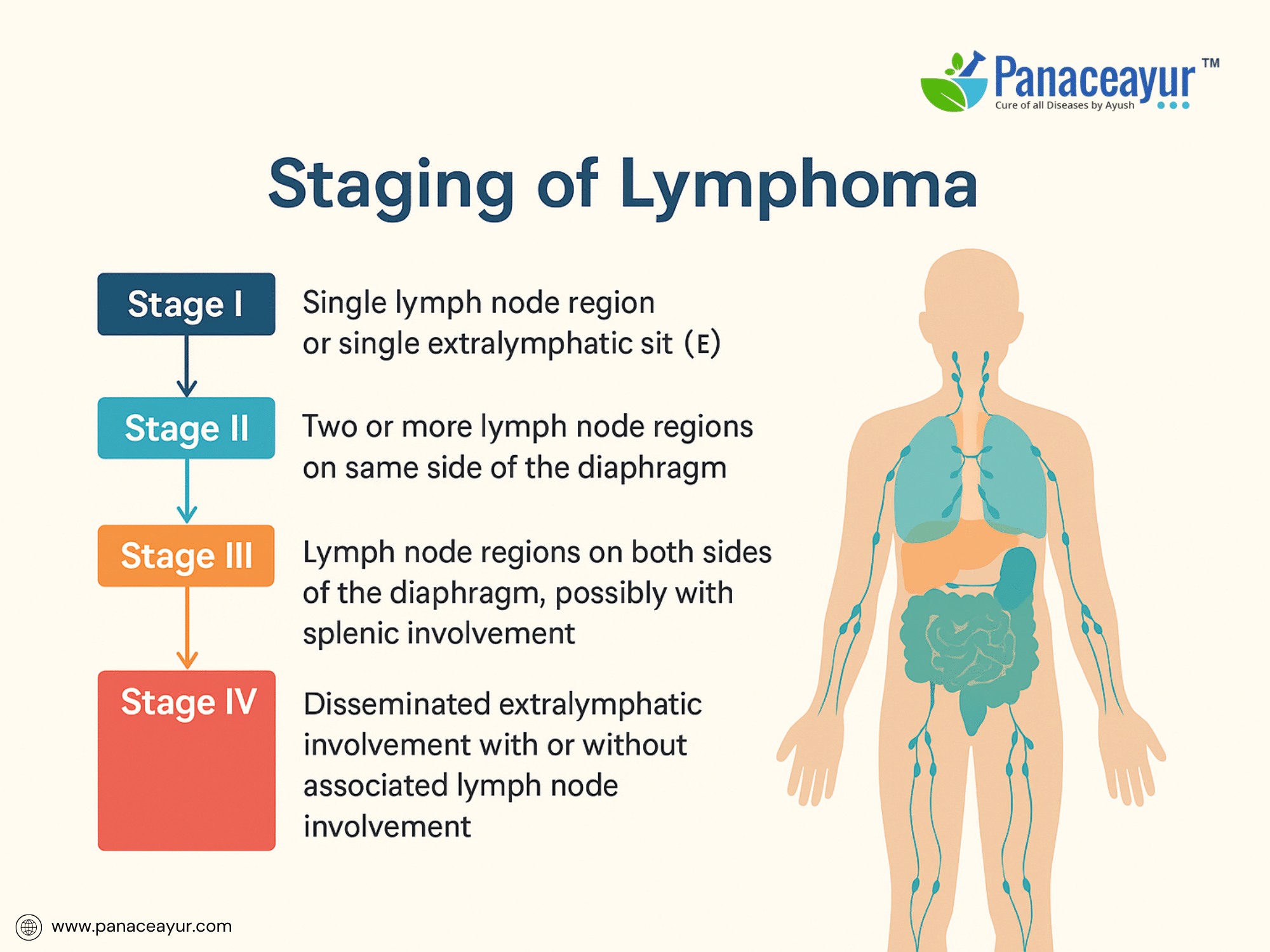
Accurate staging of lymphoma is essential for determining disease extent, guiding treatment decisions, and assessing prognosis. The universally accepted system for staging Hodgkin and Non-Hodgkin lymphoma is the Ann Arbor staging system, with modifications from the Cotswolds meeting and integration of metabolic imaging through FDG-PET/CT in recent years.
The Ann Arbor system classifies lymphoma into Stage I to IV, based on the number and location of involved lymph node regions and the presence of extranodal disease:
- Stage I: Involvement of a single lymph node region or a single extralymphatic site.
- Stage II: Involvement of two or more lymph node regions on the same side of the diaphragm.
- Stage III: Involvement of lymph node regions on both sides of the diaphragm, possibly including the spleen or localized extranodal sites.
- Stage IV: Disseminated involvement of one or more extralymphatic organs with or without lymph node involvement.
Each stage is further subclassified with A or B designations based on the presence of systemic symptoms:
- A: Absence of B symptoms.
- B: Presence of B symptoms (fever >38°C, night sweats, weight loss >10%).
Additional modifiers include:
- E: Extranodal extension contiguous with nodal disease.
- S: Splenic involvement.
In Hodgkin lymphoma, bulky disease (>10 cm nodal mass or mediastinal mass >1/3 chest diameter) is denoted as “X”, influencing treatment intensity.
Positron Emission Tomography (PET/CT) has revolutionized staging by detecting metabolically active disease sites not apparent on conventional imaging, resulting in stage migration and improved treatment planning. PET-based response criteria (Deauville 5-point scale) now supplement staging to monitor interim and end-of-treatment metabolic responses.
For Non-Hodgkin lymphoma, staging is complemented by prognostic scoring systems that integrate clinical and laboratory variables to stratify risk and predict outcomes. The most widely used is the International Prognostic Index (IPI), developed for aggressive lymphomas, incorporating five adverse factors:
- Age >60 years.
- Elevated serum lactate dehydrogenase (LDH).
- Poor performance status (ECOG ≥2).
- Ann Arbor stage III or IV.
- Involvement of >1 extranodal site.
Patients are stratified into low (0-1), low-intermediate (2), high-intermediate (3), and high-risk (4-5) categories, correlating with progressively lower survival rates. For diffuse large B-cell lymphoma, the revised IPI (R-IPI) was introduced in the rituximab era, identifying very good, good, and poor risk groups.
Other prognostic models include:
- Follicular Lymphoma International Prognostic Index (FLIPI) for follicular lymphoma.
- MIPI (Mantle Cell Lymphoma International Prognostic Index) for mantle cell lymphoma.
- Hasenclever Index for Hodgkin lymphoma.
Emerging prognostic tools incorporate molecular markers, gene expression profiling, and next-generation sequencing data, refining risk stratification beyond clinical variables. For example, detection of double-hit (MYC and BCL2/BCL6 rearrangements) or double-expressor (MYC and BCL2 protein overexpression) status in diffuse large B-cell lymphoma portends an adverse prognosis and influences therapeutic selection toward intensified regimens.
Bone marrow biopsy remains integral in staging low-grade lymphomas, while its role in Hodgkin lymphoma has diminished due to PET-CT sensitivity. Central nervous system (CNS) risk stratification using clinical models (e.g., CNS-IPI) guides prophylactic strategies in high-risk Non-Hodgkin lymphoma.
Ultimately, staging and prognostic scoring are not only pivotal for clinical decision-making but also facilitate enrollment into risk-adapted clinical trials, aligning patients with evolving personalized therapeutic approaches.
Conventional Treatment Modalities
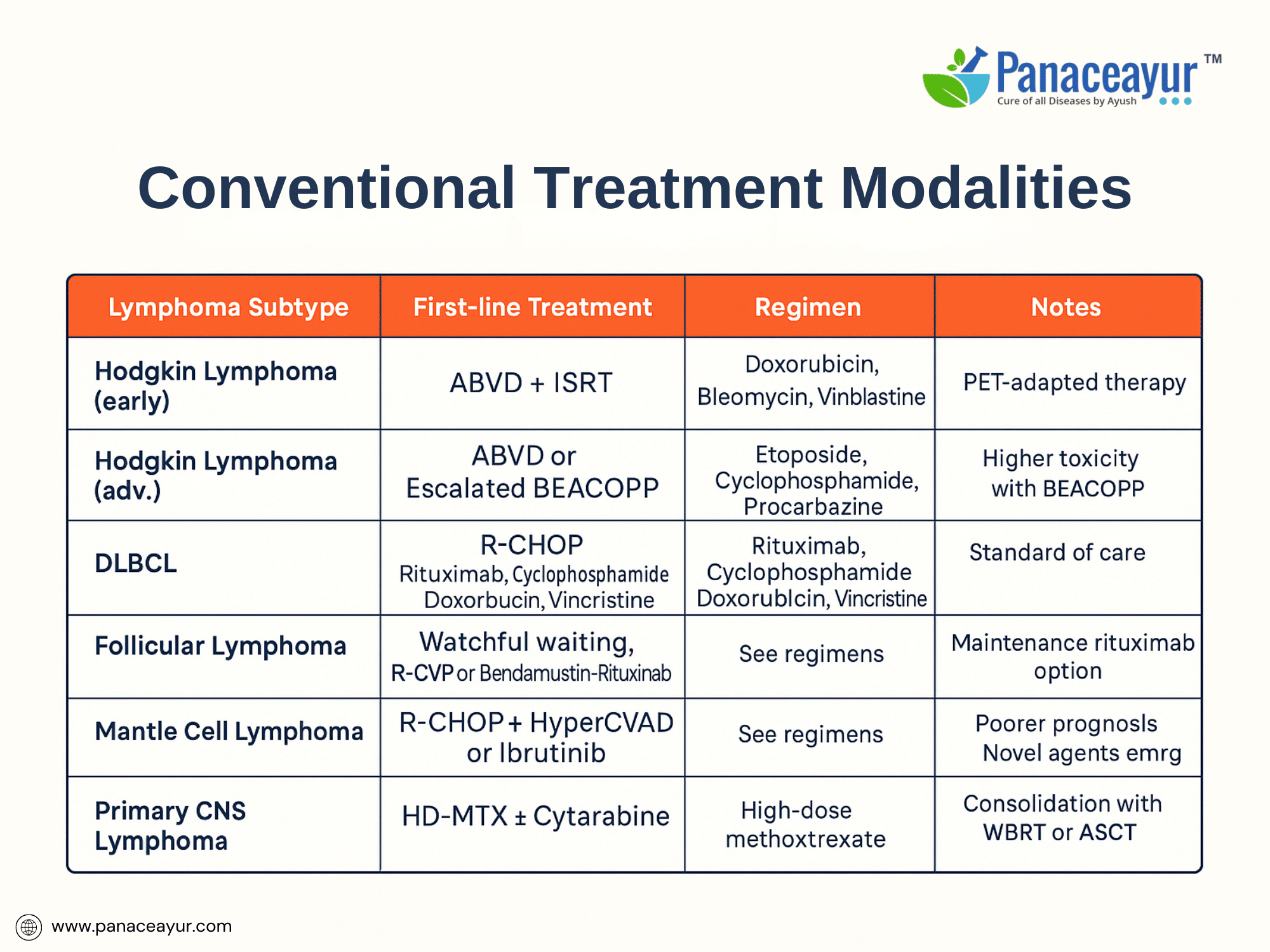
The management of lymphoma involves a multimodal therapeutic strategy, tailored to the histological subtype, disease stage, patient comorbidities, and prognostic factors. Over the past several decades, advances in chemotherapy, immunotherapy, targeted agents, and supportive care have substantially improved survival outcomes, particularly in Hodgkin lymphoma and many aggressive Non-Hodgkin lymphomas.
For Hodgkin lymphoma, the standard frontline treatment for early-stage disease is combined modality therapy, consisting of short-course chemotherapy followed by involved-site radiotherapy (ISRT). The widely adopted chemotherapy regimen is ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), administered over 2 to 4 cycles depending on risk stratification. For advanced-stage disease, 6 cycles of ABVD or escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) may be employed, with treatment intensity guided by interim PET-CT response using Deauville criteria.
In Non-Hodgkin lymphoma (NHL), treatment is highly heterogeneous, reflecting the biological diversity of subtypes. For aggressive B-cell lymphomas such as diffuse large B-cell lymphoma (DLBCL), the gold standard remains R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) administered every 21 days for 6 cycles. Rituximab, an anti-CD20 monoclonal antibody, revolutionized outcomes in CD20-positive B-cell lymphomas by enhancing progression-free and overall survival. Patients with high-risk or double-hit features may require intensified regimens (e.g., DA-EPOCH-R) or consolidation with autologous stem cell transplantation (ASCT).
Indolent lymphomas such as follicular lymphoma often follow a “watch and wait” approach in asymptomatic, low-burden disease. Treatment is initiated upon symptomatic progression or organ compromise, typically with rituximab monotherapy, R-CVP (rituximab, cyclophosphamide, vincristine, prednisone), or bendamustine-rituximab. Maintenance rituximab following induction improves progression-free survival, though its impact on overall survival remains debated.
T-cell lymphomas exhibit greater therapeutic resistance and poorer outcomes than their B-cell counterparts. Treatment regimens such as CHOP or CHOEP (adding etoposide) are commonly used, though relapse rates are high. Novel agents including brentuximab vedotin (anti-CD30 antibody-drug conjugate), pralatrexate, and histone deacetylase inhibitors (romidepsin, belinostat) provide options in relapsed or refractory settings.
Central nervous system (CNS) lymphomas, particularly primary CNS lymphoma, are managed with high-dose methotrexate-based regimens, often combined with cytarabine and rituximab, followed by consolidation with whole-brain radiotherapy or autologous stem cell transplant depending on patient age and response.
Radiotherapy remains an important modality for localized, early-stage disease, bulky residual masses, and palliation of symptomatic sites. Advances in intensity-modulated radiotherapy (IMRT) and involved-site radiation have minimized late toxicity compared to historical extended-field techniques.
Relapsed and refractory lymphomas are increasingly managed with salvage chemotherapy followed by high-dose chemotherapy with autologous stem cell rescue in eligible patients. Novel immunotherapies such as chimeric antigen receptor (CAR) T-cell therapy targeting CD19 have transformed outcomes in multiply relapsed large B-cell lymphoma, achieving durable remissions in a subset of patients.
Targeted therapies are expanding treatment options in specific molecular contexts. Ibrutinib (Bruton’s tyrosine kinase inhibitor) is approved for mantle cell lymphoma and other B-cell malignancies, while idelalisib (PI3K delta inhibitor) is approved for follicular lymphoma after multiple prior therapies. Venetoclax (BCL2 inhibitor) shows promise in double-hit and Richter’s transformation lymphomas.
Supportive care is integral to treatment success, including antimicrobial prophylaxis, tumor lysis syndrome prevention, growth factor support, fertility preservation counseling, and psychosocial interventions to mitigate treatment-related distress.
The evolving therapeutic landscape of lymphoma reflects a shift toward risk-adapted, precision medicine, where treatment is increasingly tailored to disease biology, molecular features, and individual patient factors to maximize efficacy while minimizing toxicity.
Side Effects and Long-Term Complications
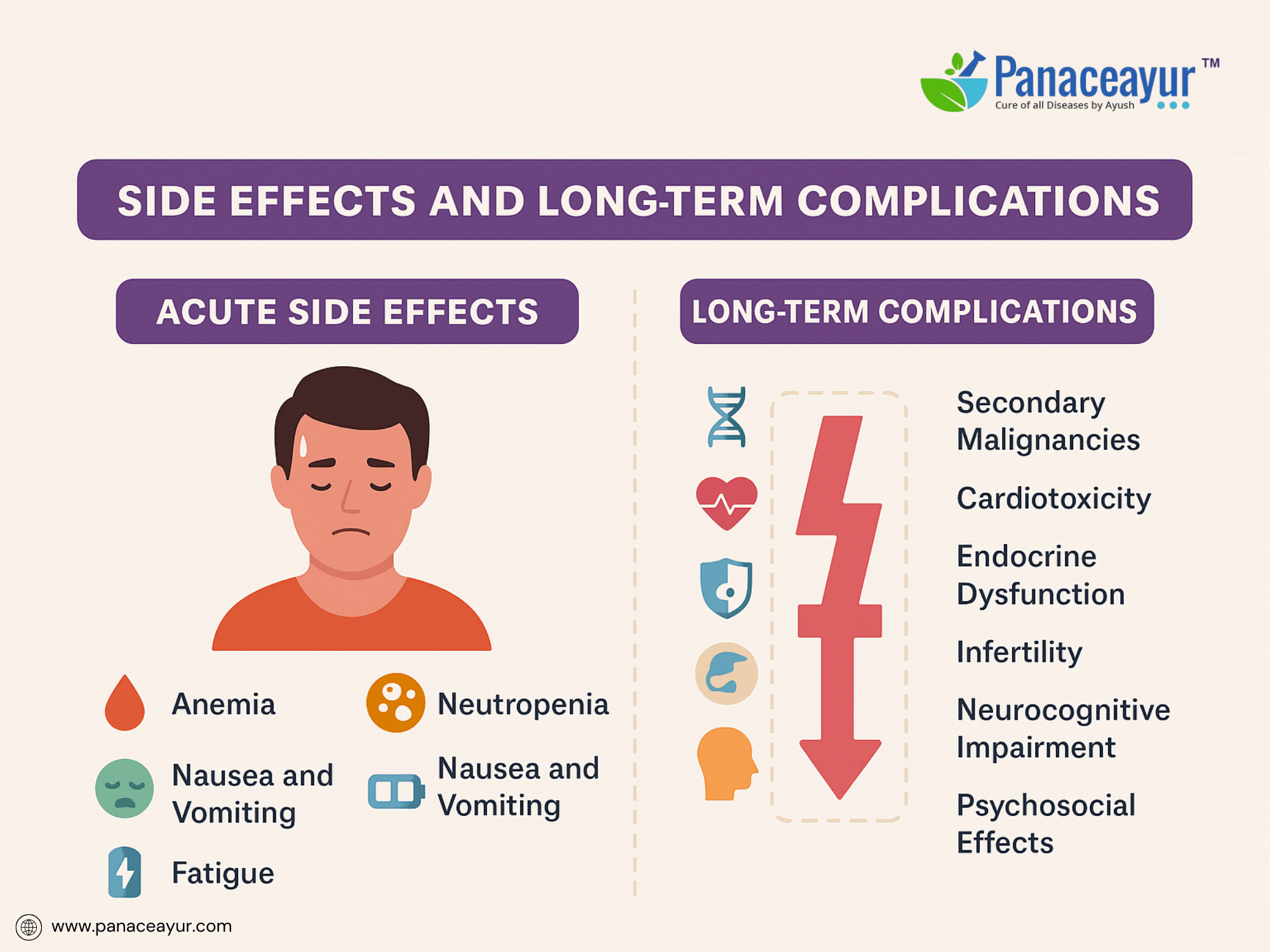
The treatment of lymphoma, while often highly effective, is associated with a spectrum of acute, subacute, and long-term toxicities that vary depending on the chemotherapy regimen, radiation exposure, targeted therapies, and individual patient susceptibility. Understanding these adverse effects is crucial for proactive management, long-term survivorship care, and patient education.
Acute side effects commonly result from cytotoxic chemotherapy and include myelosuppression (neutropenia, anemia, thrombocytopenia), leading to heightened risk for infections, bleeding, and fatigue. Patients undergoing ABVD or R-CHOP frequently experience nausea, vomiting, mucositis, alopecia, and transient liver enzyme elevations. Neutropenic fever remains a medical emergency requiring prompt empirical antibiotic therapy and supportive care. Rituximab administration may trigger infusion reactions ranging from mild chills and fevers to severe anaphylaxis, necessitating premedication with corticosteroids and antihistamines.
Intermediate-term toxicities emerge during or shortly after treatment. Peripheral neuropathy, particularly with vincristine-containing regimens, may manifest as sensory or motor deficits, impacting functional independence. Pulmonary toxicity from bleomycin, characterized by interstitial pneumonitis and pulmonary fibrosis, necessitates vigilant monitoring of respiratory symptoms and diffusion capacity. Patients receiving mantle or mediastinal radiation are at risk for esophageal strictures, hypothyroidism, and pericardial disease.
Long-term complications represent a major concern in lymphoma survivorship. One of the most significant risks is the development of secondary malignancies, including therapy-related acute myeloid leukemia (t-AML), myelodysplastic syndromes, and solid tumors such as breast, lung, or gastrointestinal cancers. Radiation-induced malignancies may occur within or adjacent to irradiated fields, with latency periods extending a decade or more post-therapy.
Cardiotoxicity is a well-documented late effect of anthracyclines (doxorubicin) and chest irradiation, leading to congestive heart failure, cardiomyopathy, or coronary artery disease. Lifelong cardiac monitoring using echocardiography or multigated acquisition (MUGA) scans is recommended for survivors exposed to cumulative anthracycline doses or thoracic radiotherapy.
Endocrine dysfunction, particularly hypothyroidism following neck irradiation and gonadal failure after alkylating agents, poses risks for infertility, sexual dysfunction, and metabolic disorders. Fertility preservation strategies, including sperm banking or oocyte cryopreservation, should be discussed with patients prior to initiating gonadotoxic therapies.
Neurocognitive deficits, encompassing attention, memory, and executive function impairments, have been reported following high-dose chemotherapy, CNS-directed therapies, or cranial irradiation, with implications for quality of life and occupational reintegration.
Psychosocial sequelae such as anxiety, depression, post-traumatic stress symptoms, and body image concerns frequently affect lymphoma survivors, underscoring the need for integrated psychosocial support and counseling.
Late infectious complications, including reactivation of hepatitis B, herpes zoster, or Pneumocystis jirovecii pneumonia, may occur in patients with prolonged B-cell depletion or corticosteroid use, warranting prophylactic antiviral or antimicrobial interventions.
In summary, the evolving paradigm of lymphoma treatment necessitates a parallel focus on toxicity mitigation, survivorship monitoring, and holistic rehabilitation to optimize long-term health outcomes. Multidisciplinary collaboration among oncologists, primary care providers, cardiologists, endocrinologists, reproductive specialists, and mental health professionals is essential to address the complex spectrum of treatment-related complications.
Ayurvedic Therapeutic Approach for Lymphoma
Lymphoma represents not only a malignancy of lymphoid tissues but a deeper disruption of immune regulation, cellular integrity, and systemic homeostasis. While modern oncology has achieved remarkable remission rates with chemotherapy and targeted therapies, many patients in the West are increasingly exploring integrative approaches to reduce side effects, improve quality of life, and address the disease from a more systemic, root-cause perspective. Ayurveda, India’s classical medical science, offers a complementary paradigm that emphasizes restoring balance across bodily systems rather than focusing solely on eradicating tumor cells.
In Ayurvedic nosology, lymphoma can be correlated with disease entities such as Granthi (nodular swelling), Arbuda (malignant tumor), and more specifically Raktarbuda (blood-related tumor), as described in texts like the Sushruta Samhita (Nidana Sthana 11/3) and Charaka Samhita (Chikitsa Sthana 7/20). Ayurvedic pathogenesis attributes the formation of abnormal masses to the interplay of vitiated Doshas (Vata, Pitta, Kapha) acting on Rakta (blood), Mamsa (muscle), and Meda (fat) tissues, leading to excessive proliferation, stagnation, and structural disorganization. Central to this process is Srotorodha (blockage of microchannels) and accumulation of Ama (toxic metabolic residues), which impair tissue metabolism, immune recognition, and clearance of abnormal cells. This framework resonates metaphorically with modern concepts of tumor microenvironment dysfunction, immune evasion, and chronic inflammation as contributors to cancer progression.
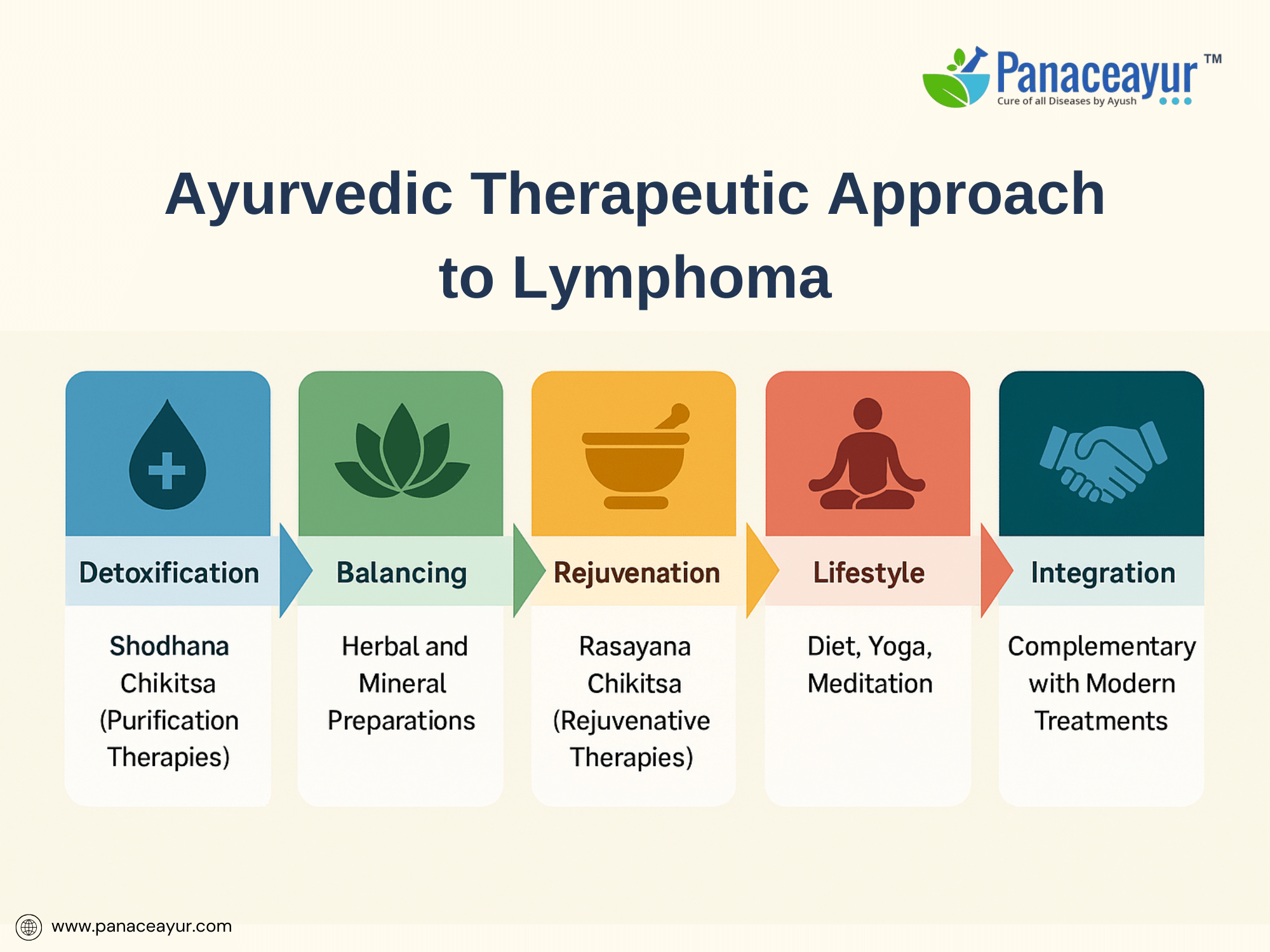
The Ayurvedic approach to lymphoma is multi-dimensional, targeting not only the visible mass but the terrain that supports abnormal growth. Treatment aims to expel pathological accumulations, arrest tumor progression, rejuvenate healthy tissues, and rebuild immune integrity. This is achieved through a combination of Shodhana Chikitsa (purification therapies), Shamana Chikitsa (palliative balancing therapies), Rasayana Chikitsa (rejuvenative therapies), dietary regulation, and mind-body practices.
Detoxification, or Shodhana, involves therapies like Virechana (therapeutic purgation) with herbs such as Trivrit (Operculina turpethum) and Haritaki (Terminalia chebula) to clear lymphatic and bile congestion, particularly useful in Kapha-Pitta dominant pathology. Basti (medicated enema therapy), using bitter decoctions in milk (Tikta Ksheera Basti), is administered to clear obstructions in the Rasavaha and Medovaha Srotas (lymphatic and fat channels). Nasya (nasal medicated drops) may be employed for cervical and supraclavicular lymphadenopathy. In selected localized cases, Raktamokshana (bloodletting) is historically described for Rakta Dushti but is cautiously adapted in modern settings. These purification measures aim to reset tissue metabolism, enhance Agni (digestive-metabolic fire), and clear pathological residues that foster tumor-supportive environments.
After detoxification, the therapeutic focus shifts to balancing therapies or Shamana, incorporating herbal and mineral medicines with documented anti-inflammatory, immunomodulatory, and anti-proliferative properties. Core herbs include Ashwagandha (Withania somnifera), known to enhance natural killer cell activity and modulate stress-induced immune suppression; Guduchi (Tinospora cordifolia), with radioprotective and DNA stabilizing properties; Guggulu (Commiphora mukul), shown to inhibit NF-κB signaling and promote apoptosis; and Haridra (Curcuma longa), whose curcumin component suppresses angiogenesis and oncogenic transcription factors. A cornerstone formulation, Kanchnar Guggulu, is traditionally prescribed for Granthi and Arbuda, combining detoxifying, anti-inflammatory, and tissue-resolving actions.
The final stage emphasizes Rasayana Chikitsa, a rejuvenative phase aimed at restoring Dhatu (tissue) integrity and Ojas (vital immune essence). Here, potent mineral preparations such as Swarna Bhasma (gold calx) and Heerak Bhasma (diamond ash) are administered under strict supervision to promote immunomodulation, cellular regeneration, and hematopoietic support. Classical Rasayanas like Gandhak Rasayan and Chyawanprash Avaleha are employed to rebuild systemic resilience, particularly after chemotherapy or radiotherapy-induced depletion. This rejuvenative phase is conceptually analogous to modern goals of post-treatment recovery and immune reconstitution, offering a holistic complement to biomedical survivorship care.
Integral to the Ayurvedic approach are dietary and lifestyle interventions. Patients are advised to follow a Kapha- and Ama-reducing diet, emphasizing bitter, astringent, and pungent tastes, while avoiding heavy, damp, processed, and mucogenic foods such as dairy and red meat. Warm, freshly prepared, digestible meals fortified with digestive spices like ginger, black pepper, and cumin support Agni and prevent further Ama accumulation. Mind-body therapies, including Yoga, Pranayama, and meditation, are prescribed to harmonize Prana (life force), alleviate emotional stressors, and support neuroendocrine balance, acknowledging the psychoneuroimmunological links now well-documented in cancer research.
Importantly, Ayurveda does not position itself in opposition to modern oncology but can be thoughtfully integrated. Contemporary research indicates potential for Ayurvedic therapies to reduce chemotherapy-induced fatigue, mucositis, and hematologic suppression while improving quality of life. A meta-analysis by Balachandran and Govindarajan (2005) reported Rasayana herbs mitigate treatment toxicity without adverse herb-drug interactions. Clinical reports from integrative oncology centers in India document regression of nodal swellings, improvement in hematological markers, and enhanced chemotherapy tolerance in patients combining Ayurvedic therapies with conventional care. However, larger controlled trials remain needed to establish efficacy within Western clinical frameworks.
Appeal
Patients seeking integrative options, Ayurveda offers a paradigm shift from attacking the tumor to restoring an internal biological terrain inhospitable to malignancy. It provides a patient-centered path that strengthens immunity, corrects underlying imbalances, promotes tissue resilience, and supports holistic healing. Patients considering Ayurvedic interventions are strongly encouraged to work with qualified Ayurvedic physicians trained in integrative oncology, ensuring safety, individualized formulation, and collaborative monitoring with their oncology team.
Prognosis and Survivorship
The prognosis of lymphoma has significantly improved over recent decades due to advances in chemotherapy, immunotherapy, and supportive care. Survival outcomes, however, remain heterogeneous and depend on multiple factors, including histological subtype, disease stage, prognostic scoring systems, molecular markers, and treatment response.
In Hodgkin lymphoma, contemporary risk-adapted protocols have achieved cure rates exceeding 85% in early-stage disease and 70–80% in advanced-stage disease. Prognosis is particularly favorable in patients with low-risk features, absence of B symptoms, normal lactate dehydrogenase (LDH), and early complete metabolic response on interim PET-CT. However, relapsed or refractory Hodgkin lymphoma remains a therapeutic challenge, often requiring salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT), with novel agents such as brentuximab vedotin and PD-1 inhibitors showing promise in refractory cases.
In Non-Hodgkin lymphoma (NHL), prognosis varies widely depending on subtype. Aggressive lymphomas like diffuse large B-cell lymphoma (DLBCL) achieve 60–70% long-term remission with R-CHOP-based therapy in first-line treatment. Favorable prognostic indicators include age <60 years, low International Prognostic Index (IPI), normal LDH, and complete metabolic remission after treatment. Conversely, high-risk features such as double-hit genetics (MYC and BCL2/BCL6 rearrangements), elevated IPI scores, and early progression portend poorer outcomes.
Indolent lymphomas such as follicular lymphoma exhibit long median survival (10–15 years) but are generally considered incurable with current therapies. The clinical course is characterized by multiple relapses and remissions, with increasing refractoriness to treatment over time. Mantle cell lymphoma, peripheral T-cell lymphoma, and other rare subtypes remain more resistant to conventional therapies, requiring novel targeted agents and investigational approaches.
Beyond survival metrics, the concept of lymphoma survivorship has gained prominence, recognizing the long-term physical, psychological, and social challenges faced by individuals post-treatment. Survivors are at risk for secondary malignancies, cardiovascular disease, endocrine dysfunction, infertility, neurocognitive decline, and psychosocial distress. Lifelong surveillance is recommended, including periodic screening for treatment-related late effects, cardiovascular monitoring, endocrine evaluations, and cancer screening tailored to prior radiation fields and chemotherapy exposures.
The transition from active treatment to survivorship necessitates a multidisciplinary approach, incorporating oncologists, primary care providers, cardiologists, endocrinologists, reproductive specialists, psychologists, and rehabilitation teams to ensure comprehensive long-term care. Education on healthy lifestyle practices, cardiovascular risk reduction, vaccination updates, and symptom monitoring empowers survivors to participate actively in their ongoing health.
From an integrative perspective, survivorship also benefits from continued use of Rasayana therapies, dietary regulation, mind-body practices, and supportive counseling, aligning with Ayurveda’s emphasis on rebuilding Ojas (vital immunity) and maintaining balance to prevent recurrence. This holistic vision of survivorship transcends mere disease-free survival, aiming for restoration of optimal physical, emotional, and spiritual well-being.
Case Study
A notable case involves a 36-year-old male diagnosed with diffuse large B-cell lymphoma (DLBCL), who presented with a rapidly enlarging cervical lymph node mass accompanied by systemic B-symptoms including fever, night sweats, and significant unintentional weight loss. Laboratory evaluation revealed elevated lactate dehydrogenase (LDH) levels, while a subsequent excisional lymph node biopsy confirmed DLBCL of the germinal center B-cell subtype. Staging with PET-CT imaging demonstrated stage III disease with mediastinal and abdominal lymph node involvement. The patient underwent frontline immunochemotherapy with the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) administered every 21 days. An interim PET scan after three cycles showed a partial metabolic response, leading to the completion of six total cycles followed by involved-field radiotherapy to residual nodal sites. The patient achieved a complete metabolic response on end-of-treatment PET imaging and maintained complete remission at a three-year follow-up, with no clinical or radiologic evidence of relapse. However, he experienced persistent mild peripheral neuropathy attributed to vincristine toxicity, underscoring the importance of monitoring long-term treatment-related side effects. This outcome aligns with findings from a large retrospective study conducted by Vaidya et al. (2012), which reported five-year overall survival rates of 75% in patients with DLBCL treated with R-CHOP, highlighting the significant therapeutic advances brought by the addition of rituximab to standard chemotherapy protocols (Vaidya, Witzig, Habermann, Maurer, & Link, 2012). The study further emphasized prognostic variability based on molecular subtype and International Prognostic Index (IPI) scores, reinforcing the need for individualized treatment strategies (https://doi.org/10.1002/ajh.23299).
In contrast to conventional protocols, emerging integrative approaches have explored the adjunctive role of Ayurvedic Rasayana therapy in lymphatic malignancies. Thatte and Dahanukar (1997) documented a case series describing the use of Rasayana herbs such as Withania somnifera and Tinospora cordifolia in patients undergoing chemotherapy for various cancers, including lymphoma. Their findings suggested improvements in immune function, appetite, treatment tolerance, and overall quality of life, though they acknowledged the need for controlled clinical trials to validate efficacy (Thatte & Dahanukar, 1997). This perspective opens avenues for integrative oncology models, where Ayurvedic interventions may complement standard care by enhancing patient resilience and mitigating side effects
(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3612316/).
References and Further Reading
Aggarwal, B. B., & Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases. The International Journal of Biochemistry & Cell Biology, 41(1), 40–59.
https://doi.org/10.1016/j.biocel.2008.06.010
Balachandran, P., & Govindarajan, R. (2005). Cancer—An ayurvedic perspective. Pharmacological Research, 51(1), 19–30.
https://doi.org/10.1016/j.phrs.2004.05.004
Mathew, S., & Kuttan, G. (1997). Antitumor activity of Tinospora cordifolia and its possible mechanism of action. Journal of Ethnopharmacology, 58(2), 83–87. https://doi.org/10.1016/S0378-8741(97)00091-3
Singh, N., Bhalla, M., de Jager, P., & Gilca, M. (2011). An overview on Ashwagandha: A Rasayana (rejuvenator) of Ayurveda. African Journal of Traditional, Complementary and Alternative Medicines, 8(5 Suppl), 208–213.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3252722/
Shishodia, S., Sethi, G., & Aggarwal, B. B. (2003). Curcumin: Getting back to the roots. Annals of the New York Academy of Sciences, 1056, 206–217.
https://doi.org/10.1196/annals.1352.010
Thakar, A. B. (2004). Ayurvedic management of benign cervical lymphadenopathy: A case report. AYU Journal, 25(4), 45–49.
https://www.ayujournal.org/text.asp?2004/25/4/45/1234
Swerdlow, S. H., Campo, E., Harris, N. L., Jaffe, E. S., Pileri, S. A., Stein, H., … & Vardiman, J. W. (2008). WHO classification of tumours of haematopoietic and lymphoid tissues (4th ed.). IARC.
National Cancer Institute. (2022). Adult Non-Hodgkin Lymphoma Treatment (PDQ®)–Patient Version. National Cancer Institute.
https://www.cancer.gov/types/lymphoma/patient/adult-nhl-treatment-pdq
Mukherjee, P. K., Wahile, A., & Saha, B. P. (2006). Indian herbal medicines for the treatment of cancer: Perspective and problems. Current Science, 90(3), 327–332.
https://www.jstor.org/stable/24091649
Khan, N., & Mukhtar, H. (2008). Multitargeted therapy of cancer by green tea polyphenols. Cancer Letters, 269(2), 269–280.
https://doi.org/10.1016/j.canlet.2008.04.014
Rao, A. V., & Agarwal, S. (2000). Role of antioxidant lycopene in cancer and heart disease. Journal of the American College of Nutrition, 19(5), 563–569.
https://doi.org/10.1080/07315724.2000.10718953
Gupta, S. C., Patchva, S., & Aggarwal, B. B. (2013). Therapeutic roles of curcumin: Lessons learned from clinical trials. The AAPS Journal, 15(1), 195–218.
https://doi.org/10.1208/s12248-012-9432-8
Sawhney, R. S., Sharma, K., & Yadav, R. S. (2017). Role of Rasayana in cancer management: A critical review. AYU, 38(3-4), 154–162.
https://doi.org/10.4103/ayu.AYU_187_16
Aggarwal, B. B., Sundaram, C., Malani, N., & Ichikawa, H. (2007). Curcumin: The Indian solid gold. Advances in Experimental Medicine and Biology, 595, 1–75.
https://doi.org/10.1007/978-0-387-46401-5_1
Wong, R. S. (2011). Apoptosis in cancer: From pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research, 30(1), 87.
https://doi.org/10.1186/1756-9966-30-87
Choudhary, N., & Bijjem, K. R. V. (2012). Chemotherapy-induced nausea and vomiting: Effect of complementary and integrative medicine. Asian Pacific Journal of Cancer Prevention, 13(12), 5913–5917.
https://doi.org/10.7314/APJCP.2012.13.12.5913
Balasubramani, S. P., Venkatasubramanian, P., & Patwardhan, B. (2011). Ayurveda and integrative oncology: Perspectives and challenges. Journal of Alternative and Complementary Medicine, 17(7), 613–616.
https://doi.org/10.1089/acm.2011.0240
Devanesan, A. A., Latha, P. G., & Remani, P. (2016). Antitumor activities of Indian medicinal plants and their phytochemicals: A review. Pharmacognosy Reviews, 10(20), 174–183.
https://doi.org/10.4103/0973-7847.194046
Hohmann, J., Molnár, J., Redei, D., Evanics, F., & Forgo, P. (2000). Antitumor activity of natural compounds from medicinal plants. Anticancer Research, 20(2B), 859–862. https://ar.iiarjournals.org/content/20/2B/859National Comprehensive Cancer Network. (2022). NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. NCCN.
https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf










One Response
This was so helpful thanks for the enlightment