High-Risk and Genetics Breast Oncology is a focused branch of cancer medicine dedicated to individuals who have a substantially elevated probability of developing breast cancer due to hereditary mutations, familial clustering, or other genetic–biological determinants [1]. It integrates oncology, medical genetics, preventive medicine, and in integrative models, Ayurveda, to enable prevention, early detection, and precision-based management [2].
Why It Matters
Breast cancer is the most commonly diagnosed cancer among women globally, and approximately 5–10% of all cases are attributable to hereditary cancer syndromes such as BRCA1, BRCA2, TP53, PTEN, and others [3]. Carriers of such pathogenic variants may face lifetime breast cancer risks exceeding 70%, with earlier onset and more aggressive subtypes than sporadic cases [4]. The “high-risk” category also includes individuals with prior high-dose chest radiation, certain histological risk lesions, and dense multi-generational family histories [5].
The Role of Genetics
Modern molecular profiling has established that germline mutations affect not only the probability of developing breast cancer but also tumor phenotype, therapeutic sensitivity, and survival outcomes [6]. For instance, BRCA1 carriers frequently present with triple-negative breast cancer, whereas BRCA2 carriers often exhibit hormone receptor-positive tumors [7]. Such insights drive personalized surveillance strategies and targeted treatment selection, including PARP inhibitor therapy in eligible cases [8].
Ayurvedic Perspective
Classical Ayurvedic sources, including the Sushruta Samhita (Nidana Sthana, Chapter 11), describe growths analogous to malignant tumors under Granthi and Arbuda [9]. These conditions arise from vitiation of Kapha and Vata doshas, leading to Dhatu dushti of Rasa, Rakta, and Mamsa Dhatus, and obstruction of Srotas [10]. Hereditary predisposition is explained through the concept of Beeja dosha (genetic imperfection in reproductive seeds), which suggests that predisposed individuals benefit from early Rasayana therapy, seasonal detoxification (Shodhana), and pathya-apathya-based lifestyle modulation to reduce disease expression [11].
Lesser-Known Facts
- Founder mutations like BRCA1 185delAG (Ashkenazi Jewish) and BRCA2 6174delT (Icelandic) create concentrated geographic clusters of high genetic risk [12].
- Male breast cancer risk is markedly elevated in BRCA2 carriers, along with increased risks for prostate and pancreatic cancers [13].
- Some BRCA1/2 carriers remain cancer-free due to protective epigenetic and lifestyle factors, which are under investigation for preventive interventions [14].
- MRI-based screening beginning at age 25 in high-risk carriers can detect lesions years before they are clinically palpable [15].
- In Ayurveda, early correction of Kapha–Vata imbalance using diet, exercise, and herbal Rasayana is viewed as crucial for genetically predisposed individuals [16].
Clinical Scope
High-Risk & Genetics Breast Oncology encompasses:
- Identification of at-risk individuals through family history and genetic testing [17]
- Risk stratification and personalized surveillance protocols [18]
- Surgical and pharmacologic prevention in selected high-risk carriers [19]
- Incorporation of germline-informed targeted therapy into modern oncologic care [8]
- Use of Ayurvedic preventive and supportive measures to enhance resilience and quality of life [11]
Genetic Risk Factors- Hereditary Syndromes and Mutations
High-Penetrance Genes
Inherited pathogenic variants in certain high-penetrance genes substantially elevate the lifetime risk of breast cancer [1]. BRCA1 and BRCA2 are the most well-recognized, accounting for up to 45–60% of hereditary breast cancer cases [2]. BRCA1 mutations are commonly linked to triple-negative breast cancer, early onset, high-grade tumors, and an increased likelihood of ovarian, pancreatic, and occasionally melanoma cancers [3]. BRCA2 mutations, in contrast, are more often associated with hormone receptor-positive breast cancer and heightened risks for ovarian, pancreatic, prostate, and male breast cancer [4]. In both cases, lifetime risk for breast cancer can exceed 70% [2]. Another high-penetrance gene, TP53, causes Li-Fraumeni syndrome, which is characterized by a markedly elevated risk for multiple cancers, including breast cancer, often before the age of 30 [5]. Because of its radiation sensitivity, carriers benefit from MRI-based screening rather than mammography [5]. PTEN mutations, associated with Cowden syndrome, predispose to breast, thyroid, and endometrial cancers [6] and are often accompanied by macrocephaly, mucocutaneous lesions, and hamartomas [6].
Moderate-Penetrance Genes
Moderate-penetrance mutations also contribute to breast cancer susceptibility [7], though to a lesser extent. CHEK2 variants are linked to a two- to threefold increased risk for breast cancer and possibly colorectal cancer, with the impact amplified by a positive family history [7]. PALB2, which works closely with BRCA2 in DNA repair, carries a lifetime breast cancer risk of up to 50–60% [8]. ATM mutations are associated with moderate risk and are sometimes tied to a higher likelihood of estrogen receptor-positive tumors [9].
Gene–Environment Interactions
The presence of a mutation does not guarantee cancer development, as environmental, hormonal, and lifestyle factors influence penetrance [10]. Reproductive history, breastfeeding duration, body mass index, alcohol use, and even shift work have all been studied as potential modifiers [10]. Ongoing research in nutrigenomics and epigenetics explores how diet, physical activity, and other exposures can alter gene expression in mutation carriers [11].
Polygenic Risk Scoring
Polygenic risk scoring (PRS) aggregates the influence of numerous common genetic variants to refine individual breast cancer risk estimates [12]. In carriers of high- or moderate-risk genes, PRS can shift the predicted lifetime risk upward or downward, potentially affecting surveillance recommendations and the timing of preventive surgeries [12]. This tool is particularly valuable for moderate-risk carriers, where management guidelines are less definitive [12].
Ayurvedic Perspective
In Ayurveda, hereditary cancer risk is understood through the concept of Beeja dosha, an inherent flaw in the reproductive seed material that predisposes individuals to disease [13]. Classical guidance emphasizes preconception care (Garbhini Paricharya) to strengthen the genetic foundation [13], Rasayana therapy to maintain genetic integrity [13], and seasonal detoxification to reduce the likelihood of latent risk manifesting [13]. Dietary discipline, lifestyle balance, and tailored herbal support are seen as key modulators of inherited susceptibility, aligning with modern perspectives on gene–environment interaction [14].
Risk Modifiers Across the Life Course
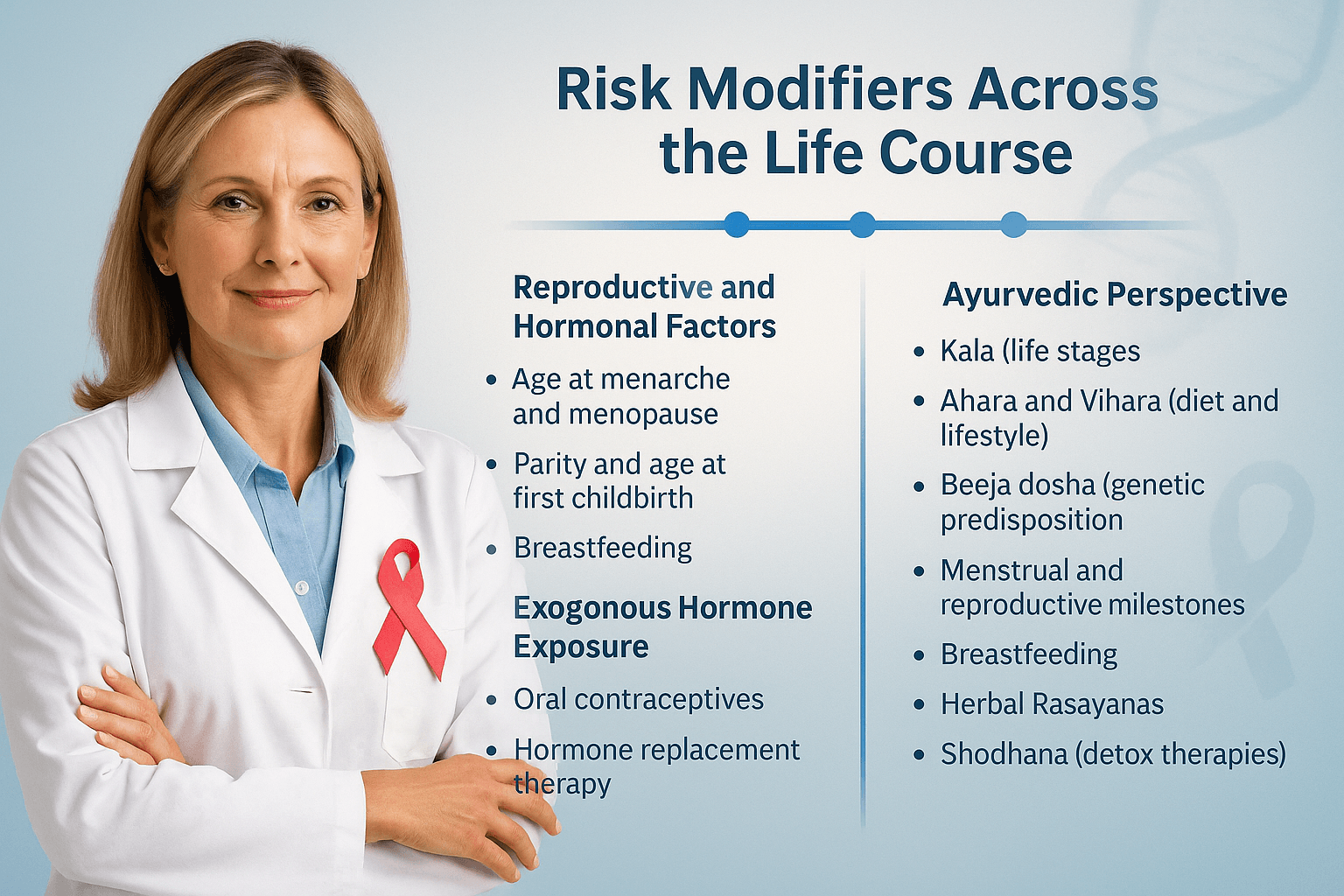
Breast cancer risk in high-risk genetic carriers is not static; it is influenced by factors that change over a person’s lifespan. Understanding these risk modifiers is essential for tailoring prevention, screening, and lifestyle advice for BRCA1, BRCA2, TP53, and other mutation carriers [1].
Reproductive and Hormonal Factors
- Age at menarche and menopause: Early menarche (<12 years) and late menopause (>55 years) extend lifetime estrogen exposure, modestly increasing breast cancer risk in mutation carriers [2].
- Parity and age at first childbirth: Multiple full-term pregnancies and early age at first childbirth tend to lower risk in the general population, but the effect in BRCA carriers is more nuanced. For BRCA1, early first childbirth may confer protection; for BRCA2, the relationship is less consistent [3].
- Breastfeeding: Observational studies suggest that breastfeeding for ≥12 months cumulatively can reduce breast cancer risk in BRCA1 carriers by up to 30% [4].
- Pregnancy after breast cancer: Current evidence shows no adverse effect on maternal prognosis for mutation carriers who conceive after treatment, provided appropriate disease-free intervals are observed [5].
Exogenous Hormone Exposure
- Oral contraceptives (OCPs): In BRCA1/2 carriers, OCPs are associated with a reduced ovarian cancer risk but a complex breast cancer risk profile—potentially higher in BRCA1 carriers with early (<20 years) long-term use, but not consistently in older users [6].
- Hormone replacement therapy (HRT): After risk-reducing salpingo-oophorectomy (RRSO), short-term estrogen-only HRT may be acceptable in selected BRCA1 carriers to mitigate menopausal symptoms without significantly increasing breast cancer risk [7].
Lifestyle and Environmental Factors
- Body weight and physical activity: Maintaining a healthy BMI and regular moderate-to-vigorous physical activity have been linked to lower breast cancer risk and improved survivorship in both high-risk and general populations [8].
- Alcohol intake: Even moderate alcohol consumption can increase breast cancer risk, and this effect may be additive in mutation carriers [9].
- Dietary patterns: Diets high in plant-based foods, fiber, and omega-3 fatty acids are associated with favorable hormonal and inflammatory profiles, which may indirectly reduce risk [10].
Medical Interventions
- Risk-reducing surgery timing: The protective benefit of bilateral prophylactic mastectomy and RRSO is most pronounced when performed before natural menopause for BRCA carriers [11].
- Chemoprevention: Selective estrogen receptor modulators (SERMs) and aromatase inhibitors can reduce breast cancer incidence in high-risk women; data in mutation carriers are more robust for BRCA2 and ER-positive phenotypes [12].
Ayurvedic Perspective
Ayurveda interprets these modifiers through the lens of Kala (life stages), Ahara (diet), Vihara (lifestyle), and Beeja dosha (genetic predisposition) [13].
- Menstrual and reproductive milestones are considered critical windows when Rasa Dhatu (plasma/lymph) and Rakta Dhatu (blood) are especially susceptible to Dosha vitiation.
- Breastfeeding is valued not only for infant health but also for maternal Stanya Shodhana (cleansing of breast channels) and Kapha balance, aligning with observed protective effects.
- Herbal Rasayanas such as Guduchi (Tinospora cordifolia), Shatavari (Asparagus racemosus), and Amalaki (Emblica officinalis) are traditionally prescribed during post-partum and perimenopausal transitions to restore Dhatu integrity and Ojas.
- Seasonal Shodhana (detox therapies) and Panchakarma—tailored to Prakriti—are viewed as preventive measures during hormonally sensitive periods.
Lesser-Known Clinical Insights
- Physical activity post-diagnosis may improve disease-free survival in BRCA carriers as much as in sporadic cases [8].
- Night-shift work and circadian rhythm disruption have been hypothesized to increase risk through melatonin suppression, though data are inconsistent [14].
- BRCA1 carriers may be more sensitive to reproductive history modifiers than BRCA2 carriers, influencing personalized counseling [3].
Risk Assessment and Stratification
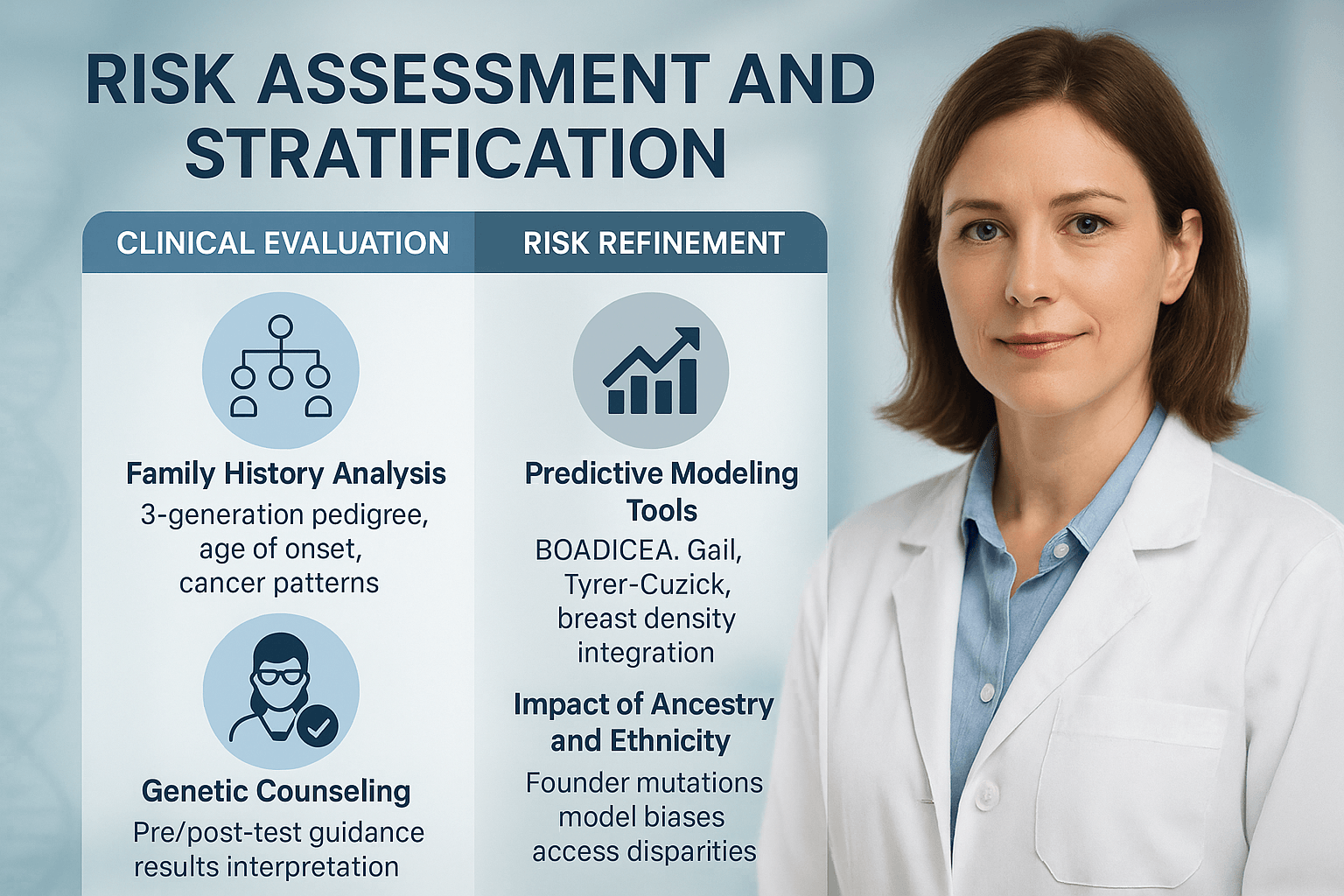
Family History Analysis
A detailed family history remains one of the most important tools in identifying individuals at high risk for breast cancer [1]. Clinicians are advised to construct a three-generation pedigree, documenting both maternal and paternal lineages. Information should include cancer type, age at diagnosis, current age or age at death, and the presence of bilateral or multiple primary cancers [2]. Patterns suggestive of hereditary syndromes include early-onset breast cancer, ovarian cancer in the family, multiple affected relatives, and cases of male breast cancer [3]. This information not only guides genetic testing eligibility but also helps determine the intensity of surveillance strategies [4].
Genetic Counseling Process
Genetic counseling is a critical step before and after genetic testing for high-risk breast cancer genes [5]. Pre-test counseling ensures that individuals understand the implications of results, including potential psychological effects, medical management changes, and possible insurance or employment concerns [6]. Post-test counseling provides interpretation of findings, whether pathogenic variants, likely pathogenic variants, or variants of uncertain significance (VUS) [7]. Counseling also facilitates cascade testing for at-risk relatives and helps families make informed decisions about preventive interventions [8].
Predictive Modeling Tools
Risk prediction models integrate personal and family history data, and in some cases, breast density and genetic test results, to estimate an individual’s lifetime risk of developing breast cancer [9]. BOADICEA, Gail Model, and Tyrer–Cuzick are among the most widely used tools [10].
- BOADICEA incorporates extensive pedigree data and can integrate genetic testing and polygenic risk scores, making it highly applicable in hereditary cancer clinics [11].
- Gail Model uses limited family history data but includes reproductive and hormonal factors, making it suitable for population-based screening recommendations [12].
- Tyrer–Cuzick Model combines detailed family history, hormonal factors, and breast density, offering accurate lifetime risk estimates for many patients [13].
Impact of Ancestry and Ethnicity
An individual’s ancestry can significantly influence both genetic mutation prevalence and the accuracy of risk models [14]. Certain founder mutations, such as BRCA1 185delAG and 5382insC in Ashkenazi Jewish populations or BRCA2 999del5 in Iceland, occur at much higher frequencies in specific ethnic groups [15]. Models that have not been validated in non-European populations may underestimate or overestimate risk [16]. Additionally, disparities in access to testing and counseling persist across ethnic and socioeconomic groups, underscoring the need for culturally adapted screening strategies [17].
Symptoms and Early Warning Signs
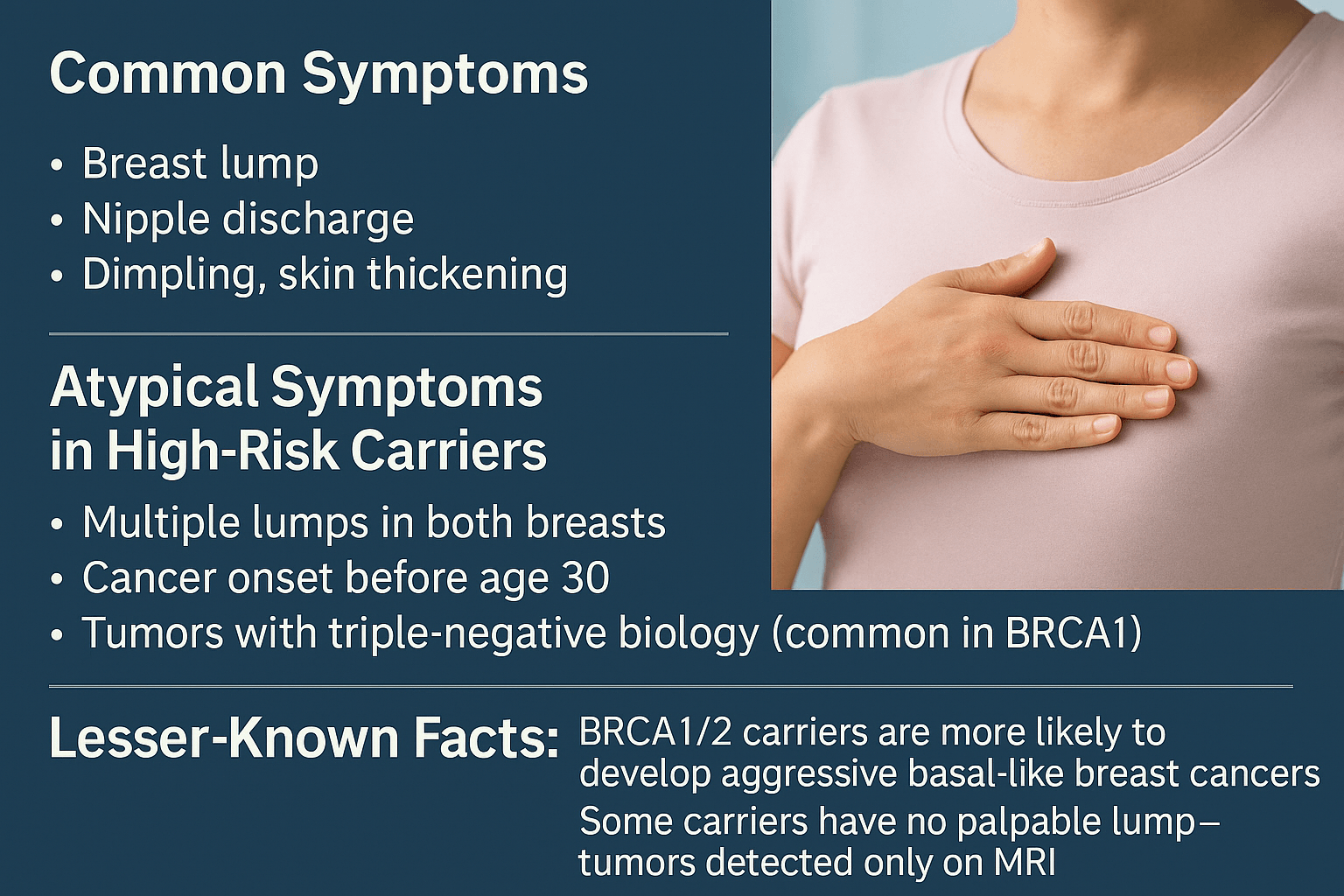
Early recognition of breast cancer symptoms in high-risk individuals is vital because tumors associated with hereditary mutations often progress more rapidly and present at a younger age than sporadic cases [1]. This section outlines both the common signs that overlap with general breast cancer presentations and the atypical patterns more frequently observed in mutation carriers, along with lesser-known clinical insights that are critical for accurate and timely diagnosis.
Common Symptoms
In both high-risk and average-risk populations, the most prevalent symptom of breast cancer is the development of a new palpable lump in the breast [2]. In hereditary cases, these lumps are often firm, irregular, and fixed to surrounding tissue, reflecting invasive growth patterns [3]. A lump may arise in the breast or, in some cases, in the axillary or supraclavicular regions as a sign of lymph node involvement [4].
Nipple discharge is another significant warning sign, especially when it occurs spontaneously, from a single duct, and contains blood or is serous in nature [5]. Discharge that is associated with a lump warrants urgent imaging and histological evaluation.
Skin changes such as dimpling, puckering, localized thickening, erythema, or an orange-peel appearance (peau d’orange) may indicate dermal lymphatic invasion or tumor infiltration into the Cooper’s ligaments [6]. These changes may be localized or diffuse and can sometimes be misattributed to benign skin conditions, especially in early stages.
Atypical Symptoms in High-Risk Carrier
In carriers of BRCA1, BRCA2, TP53, and other pathogenic variants, the clinical presentation can deviate significantly from the classic patterns [7]. One such presentation is the occurrence of multiple synchronous lumps in one or both breasts, suggesting multicentric or bilateral disease [8]. This is particularly important because bilateral involvement may be missed if clinicians focus only on the symptomatic side.
Early-onset disease is another hallmark, with some carriers developing invasive cancer before the age of 30 [9]. This early presentation often coincides with higher-grade histopathology, rapid proliferation indices, and reduced responsiveness to hormone therapies [10].
A particularly aggressive subtype, triple-negative breast cancer (TNBC), is disproportionately represented in BRCA1 mutation carriers [11]. TNBC lacks estrogen receptor, progesterone receptor, and HER2 expression, limiting targeted therapy options and necessitating prompt initiation of chemotherapy. These tumors tend to metastasize earlier and more widely, especially to visceral organs and the brain [12].
In TP53 mutation carriers (Li-Fraumeni syndrome), breast cancers may arise as part of a multi-malignancy pattern and can present alongside sarcomas or brain tumors. In these patients, standard mammography is often avoided due to radiation sensitivity, increasing the reliance on MRI for early detection [13].
Lesser-Known Facts
Epidemiological studies show that BRCA1 and BRCA2 carriers are more likely to develop basal-like breast cancers—a molecular subtype characterized by the expression of basal cytokeratins (CK5/6, CK14) and epidermal growth factor receptor (EGFR) [14]. These tumors are typically high-grade, rapidly growing, and associated with poorer prognosis if detected late.
Importantly, not all carriers will present with a palpable lump. In many cases, especially among women enrolled in high-risk screening programs, tumors are detected solely via MRI before they become clinically apparent [15]. This underscores the limitations of relying solely on physical examination or even standard mammography in younger high-risk women, as dense breast tissue can mask small lesions [16].
Another underappreciated fact is that certain hereditary cancers may exhibit unusual metastatic patterns. For instance, BRCA1/2-associated breast cancers can occasionally metastasize to the ovaries or pancreas—sites that are also at increased primary cancer risk in these mutation carriers [17]. Such patterns have implications for both surveillance and staging at diagnosis.
Clinical Implications
For high-risk carriers, the threshold for diagnostic workup should be significantly lower than in the general population. Even subtle asymmetry, faint skin changes, or unexplained nipple alterations should prompt advanced imaging, ideally with MRI [18]. This approach not only increases the chances of early-stage detection but also enables the timely initiation of targeted therapy or surgical planning that can influence long-term outcomes.
Equally, patient education plays a pivotal role. High-risk individuals should be taught how to recognize both common and atypical signs and be encouraged to report any changes promptly, even if they seem minor or transient [19]. A proactive surveillance culture among mutation carriers can mean the difference between early, curable disease and advanced, less treatable cancer.
Risk Assessment & Diagnosis
Risk Assessment
A thorough and methodical risk assessment is the foundation for early detection and prevention in breast cancer, especially among individuals with genetic susceptibility. This begins with a detailed family history mapping over three generations, capturing information about both maternal and paternal relatives, ages at diagnosis, and types of malignancies. Such mapping helps identify hereditary patterns, syndromic associations, and overlapping cancer risks that might otherwise go unnoticed [1].
Genetic counseling plays a critical role before and after testing. Pre-test counseling provides education on the implications of positive or negative results, the psychological impact, and the range of possible medical interventions, from enhanced surveillance to prophylactic surgery. Post-test counseling ensures that results are interpreted in a personalized context, incorporating factors such as age, comorbidities, and personal reproductive plans [2].
Several predictive modeling tools—including the Gail Model, Tyrer-Cuzick, and BOADICEA—quantify breast cancer risk by integrating demographic, hormonal, reproductive, and family history variables. While the Gail Model is widely used in general populations, Tyrer-Cuzick and BOADICEA offer greater precision for high-risk groups, especially those with BRCA1/2 or PALB2 mutations [3]. The choice of model can influence screening intervals, imaging modalities, and chemoprevention eligibility.
Diagnostic Tools
Modern diagnostic approaches are multi-layered, combining imaging with molecular profiling. Mammography remains the frontline tool, especially for women over 40, but is often complemented by MRI for younger women and high-risk mutation carriers due to its higher sensitivity in dense breast tissue [4]. Ultrasound serves as both an adjunct to mammography and a primary tool in younger women or those who cannot undergo MRI. Digital breast tomosynthesis (3D mammography) provides improved lesion detection by reducing tissue overlap artifacts [5].
Beyond imaging, tumor genomic profiling allows for molecular subtype identification (e.g., triple-negative, HER2-positive), which directly informs treatment strategies and prognosis. Liquid biopsy, which detects circulating tumor DNA (ctDNA), is emerging as a minimally invasive tool for early relapse detection and real-time monitoring of treatment response [6].
Ayurvedic Diagnostic Perspective
In Ayurveda, breast conditions are mapped to Granthi (benign swellings) and Arbuda (malignant tumors) as described in the Sushruta Samhita – Nidana Sthana, where causation is linked to Dosha vitiation and impaired tissue metabolism [7]. For breast malignancies, there is often a predominance of Kapha and Pitta doshas, leading to stagnation and inflammation.
The assessment focuses on Dhatu Dushti—especially Rasa Dhatu (plasma/lymph), Rakta Dhatu (blood), and Mamsa Dhatu (muscle tissue)—as these are directly involved in tumor formation. Srotas blockage (micro-channel obstruction), particularly in Raktavaha and Mamsavaha Srotas, is evaluated as a pathogenic driver. Palpation, visual examination, patient history, and pulse analysis (Nadi Pariksha) help identify the dominant dosha involvement and guide individualized treatment planning [8].
Lesser-Known Fact
Liquid biopsy has shown the potential to detect circulating tumor DNA in high-risk carriers years before any changes are visible on imaging. This could revolutionize early intervention strategies by triggering preventive or therapeutic actions at the molecular stage, long before a tumor becomes radiologically evident [9].
Modern Risk Tools – Beyond the 20% Lifetime Risk Threshold
In the past, women were considered “high risk” for breast cancer if their estimated lifetime risk was 20% or higher, often calculated with models such as Gail or Claus. Modern oncology now takes a more precise, multi-factor approach, integrating genetic, molecular, hormonal, and environmental data.
Polygenic risk scores (PRS)
PRS evaluate hundreds to thousands of common genetic variants (SNPs), not just BRCA1 or BRCA2, to give a more personalized risk estimate. This is useful for those without high-impact mutations but with strong family histories.
Integrated models (e.g., BOADICEA, CanRisk)
These combine family history, rare high-impact mutations (BRCA1, BRCA2, PALB2, TP53, CHEK2, ATM), common low-impact SNPs, hormonal and reproductive history, and lifestyle factors such as BMI, alcohol intake, and physical activity.
Hormonal and receptor status prediction
Some models estimate not just the overall risk but also the likely breast cancer subtype (ER+, triple-negative, HER2+), guiding preventive strategies and early surveillance.
Imaging-based AI predictors
Artificial intelligence tools applied to mammograms or MRIs can detect subtle tissue patterns and density changes years before diagnosis, offering a risk estimate that changes with age and hormonal status.
Liquid biopsy-based early alerts
Circulating tumor DNA and microRNA profiles are being explored to identify pre-clinical molecular signs of cancer in high-risk individuals—sometimes years before imaging shows abnormalities.
Ayurvedic integrative perspective
Ayurvedic assessment considers constitutional tendencies (Prakriti), tissue susceptibility (Dhatu Dushti)—especially Rasa Dhatu (nutritive fluid), Rakta Dhatu (blood), and Mamsa Dhatu (muscle)—and Srotas (microchannel) integrity. A Pitta-Kapha constitution with Rasa-Rakta Dushti is described in Sushruta Samhita as more prone to Arbuda (malignant growth).
Key takeaway
Instead of relying on a fixed 20% lifetime risk cutoff, modern and integrative approaches map risk dynamically, combining genetics, biology, lifestyle, and Ayurvedic constitution for personalized prevention and early detection.
Genetic Testing Workflow Updates
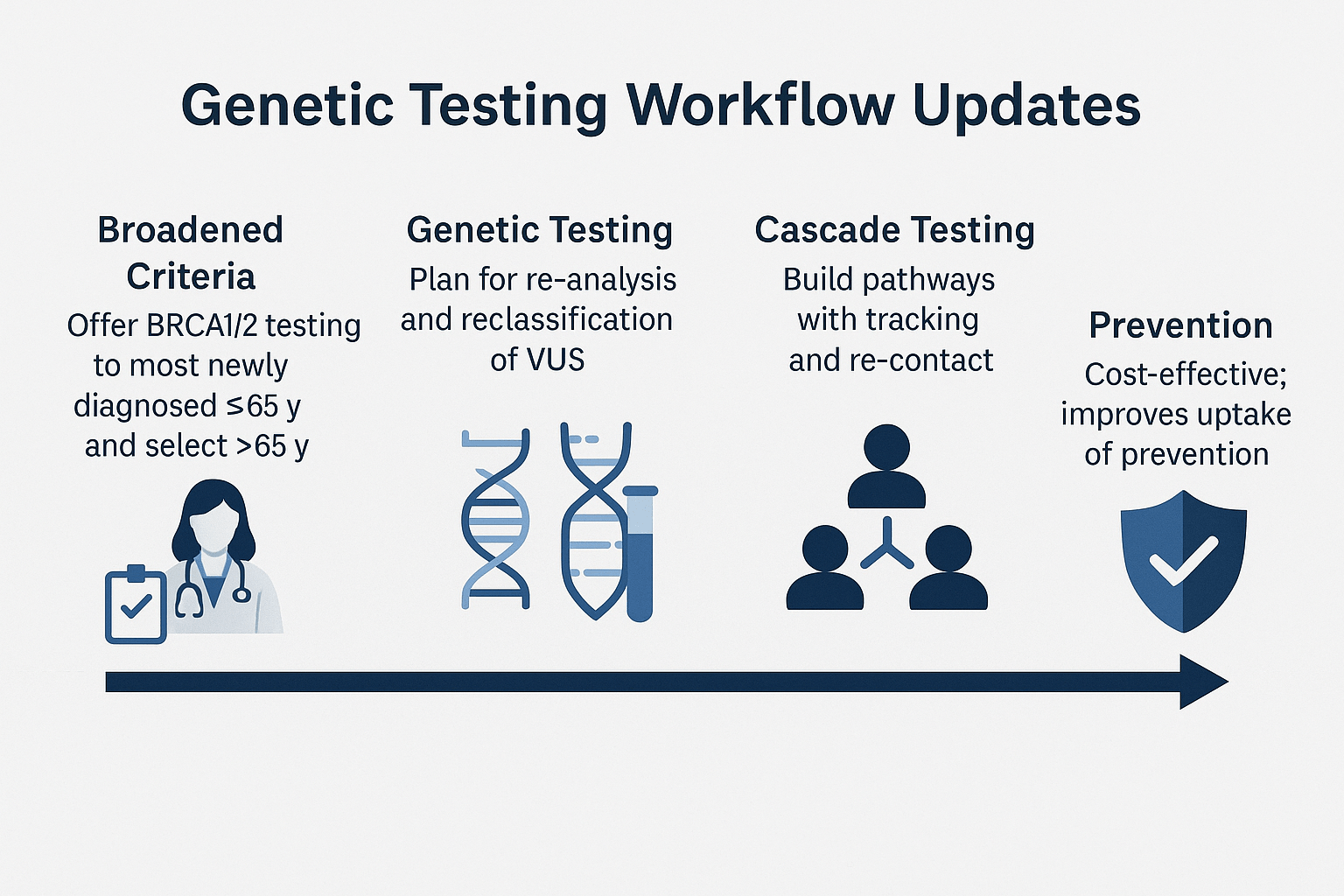
Broadened testing criteria
Recent guidelines, including the ASCO/SSO 2024 recommendations, have expanded the eligibility for germline BRCA1 and BRCA2 testing [1]. Testing is now advised for most newly diagnosed breast cancer patients aged 65 years or younger, regardless of family history. For those over 65, testing is recommended when there are clinical features suggestive of hereditary risk, such as triple-negative disease, strong family history, or multiple primary cancers [2]. This shift aims to identify more carriers who could benefit from targeted therapy and preventive strategies.
Re-analysis and VUS reclassification
Genetic data interpretation is dynamic. A variant of uncertain significance (VUS) today may be reclassified as pathogenic or benign as more evidence accumulates. Laboratories and clinicians are encouraged to implement re-analysis protocols every 1–2 years, and patients should be informed about the importance of re-contact for updated results [3]. This ensures that carriers receive timely access to preventive measures or treatment adjustments based on the most current genomic understanding.
Cascade testing implementation
Cascade testing—offering targeted genetic testing to biological relatives of individuals with known pathogenic variants—remains one of the most effective and cost-efficient public health interventions in hereditary cancer prevention [4]. Successful programs include a tracking system for at-risk relatives, structured communication support, and reminders for follow-up. Studies have shown that organized cascade testing significantly increases uptake among family members, enabling earlier interventions such as enhanced screening or prophylactic surgery [5].
Integrated follow-up systems
Best practice now includes building a genetic testing workflow that not only identifies mutation carriers but also connects them to a dedicated care pathway. This may involve genetic counselors, oncology nurses, and digital health platforms that facilitate result sharing, family outreach, and educational resources [6].
Preventive Strategies
Ayurvedic-focused prevention
In Ayurveda, cancer prevention for genetically predisposed individuals begins with Rasayana therapy to fortify Ojas (vital immunity) and maintain dosha balance. Classical Rasayanas such as Amalaki (Emblica officinalis), Ashwagandha (Withania somnifera), and Guduchi (Tinospora cordifolia) are valued for enhancing cellular resilience, neutralizing oxidative stress, and reducing chronic inflammation. Alongside these herbs, mineral-based formulations like Swarna Bhasma (calcined gold), Rajata Bhasma (calcined silver), Abhraka Bhasma (calcined mica), and Heerak Bhasma (diamond ash) are prescribed in micro-doses to strengthen tissue regeneration and prevent malignant transformation.
Classical reference:
“Balaṁ dehānāṁ vardhayati, doṣa-vṛddhi-vināśanam |
jarāmṛtyu-vinirmuktaṁ, ayuḥ śatam anuvrajet”
(Chikitsa Sthana, Charaka Samhita, Rasayana Adhyaya)
— “It enhances the strength of the body, destroys dosha aggravation, and helps one live a full lifespan free from decay and premature death.”
Pathya-Apathya guidelines emphasize fresh, seasonal, and easily digestible food, avoiding stale, processed, and toxin-producing items to prevent Ama accumulation. Seasonal Shodhana (detoxification), including Virechana (purgation) and Rakta Mokshana (bloodletting), is advised for at-risk individuals to maintain purity of dhatus (tissues) and prevent malignant dosha vitiation.
Modern measures
Risk-reducing surgeries such as prophylactic mastectomy and salpingo-oophorectomy remain key preventive options for BRCA mutation carriers. Chemopreventive agents like selective estrogen receptor modulators (SERMs) and aromatase inhibitors are also available but require careful benefit–risk analysis.
Lifestyle integration
A plant-based, antioxidant-rich diet, intermittent fasting for metabolic reset, and consistent stress reduction through yoga, pranayama, and meditation complement both Ayurvedic and modern protocols.
Lesser-known insight
Some BRCA carriers who breastfed for over 12 months have shown a reduced incidence of breast cancer in observational studies, indicating that even in high-risk populations, certain natural behaviors can have a measurable preventive impact.
Management & Ayurvedic Cure

Modern medical management of BRCA-mutated cancers often combines targeted and systemic approaches. PARP inhibitors selectively exploit the defective DNA repair mechanisms in BRCA-positive tumors, leading to synthetic lethality and tumor regression [1]. Platinum-based chemotherapeutic agents such as cisplatin and carboplatin remain standard due to their DNA crosslinking effect, which is particularly effective in BRCA-related malignancies [2]. For tumors with PD-L1 expression, immunotherapy with immune checkpoint inhibitors may improve overall survival by restoring cytotoxic T-cell activity [3]. While these therapies can extend progression-free survival, recurrence and systemic toxicity remain major concerns, making integrative strategies essential.
Ayurvedic management addresses not only the tumor but also the patient’s systemic resilience, focusing on correcting deranged doshas, cleansing obstructed channels, and rejuvenating dhatus. In selective cases, Shodhana therapies such as Virechana (therapeutic purgation) are employed to eliminate excess Pitta and Rakta vitiation, while Raktamokshana (bloodletting) is used for localized inflammatory congestion [4].
Following purification, Rasayana therapy is central for immune restoration and metabolic correction. Classical Rasayanas such as Ashwagandha (Withania somnifera) enhance Ojas and exhibit proven immunomodulatory and anti-proliferative effects [5]. Shatavari (Asparagus racemosus) nourishes Rasa and Shukra Dhatu, aiding hormonal balance and tissue regeneration [6]. Amalaki (Emblica officinalis), revered in Charaka Samhita for its Vayasthapana (age-sustaining) property, is a rich antioxidant and Pitta-pacifier with demonstrated cytoprotective effects [7]. Kanchnar Guggulu (Bauhinia variegata + Commiphora mukul) is described in Bhaishajya Ratnavali – Granthi Apachit Prakarana for dissolving abnormal growths (Granthi, Apachi) and has been shown in modern studies to possess anti-proliferative and apoptosis-inducing activity [8].
Mineral preparations are reserved for specialist-supervised protocols due to their potency. Swarna Bhasma (calcined gold) acts as a cellular rejuvenator with DNA repair enhancing properties [9]. Abhrak Bhasma (purified mica) supports tissue oxygenation and mitochondrial efficiency [10]. Heerak Bhasma (diamond ash) is considered a supreme Rasayana, believed to stabilize cellular membranes and restore immune surveillance [11].
Mind–body interventions such as Satvavajaya Chikitsa (psychological resilience therapy), Yoga, and Pranayama aim to regulate neuroendocrine function, reduce cortisol-driven immune suppression, and enhance treatment tolerance [12].
Classical support for this integrated approach is found in Charaka Samhita, Chikitsa Sthana 1/4, where it is stated:
“Rasayanam cha tat jneyam yat jaravyadhi-nashanam, ayushya-balavarnaya smriti-medha-agni-prajasthapanam.”
(That is known as Rasayana which destroys aging and disease, enhances life, strength, complexion, memory, intellect, digestion, and progeny).
Lesser-known insight: While Kanchnar Guggulu is primarily documented for glandular swellings and fibrotic growths, observational use in BRCA-positive women with benign ovarian cysts has shown reduction in cyst size and recurrence risk when combined with seasonal detox and Rasayana support, although controlled trials are needed [13]
FAQs
1. What is the main advantage of combining modern oncology with Ayurveda in BRCA-related cancers?
Modern treatments like PARP inhibitors and platinum-based chemotherapy target cancer cell pathways, while Ayurveda addresses the root cause through Shodhana (cleansing), Rasayana (rejuvenation), and immune restoration. This dual approach may enhance overall survival, reduce recurrence, and improve quality of life.
2. Can Ayurveda alone cure BRCA-positive cancer?
Ayurveda offers curative potential in certain cases when disease is detected early and protocols are followed strictly under expert guidance. However, integration with modern oncology can be crucial for aggressive or advanced stages.
3. What Ayurvedic herbs and minerals are most important for BRCA-related cancers?
Key herbs include Ashwagandha, Amalaki, Shatavari, and Kanchnar Guggulu. Mineral preparations like Swarna Bhasma, Abhrak Bhasma, and Heerak Bhasma are used under strict supervision for their Ojas-enhancing and anti-cancer properties.
4. Is Kanchnar Guggulu effective for tumor reduction?
Yes. Ayurvedic classics describe Kanchnar Guggulu for dissolving abnormal growths (Arbuda), and modern research supports its anti-proliferative and anti-inflammatory activity.
5. Can Shodhana therapy be done for all BRCA-positive individuals?
No. Shodhana (like Virechana or Raktamokshana) is recommended only after careful assessment of strength, stage, and dosha involvement.
6. What dietary rules are important for BRCA mutation carriers according to Ayurveda?
Follow Pathya-Apathya guidelines: avoid excessive processed food, red meat, and refined sugar. Favor seasonal vegetables, whole grains, ghee in moderation, and spices like turmeric and cumin for anti-inflammatory support.
7. Is breastfeeding protective against breast cancer in BRCA carriers?
Some observational studies suggest over 12 months of breastfeeding may reduce risk in BRCA carriers, though this should be considered alongside other risk-reduction measures.
8. Can Ayurvedic medicines interact with chemotherapy or PARP inhibitors?
Yes, certain herbs and minerals may enhance or interfere with drug action. Always disclose your Ayurvedic regimen to your oncologist and work with an integrative medical team.
9. What lifestyle practices are most beneficial in Ayurveda for cancer prevention?
Daily Yoga, Pranayama, meditation, early sleep, exposure to morning sunlight, and seasonal detox are key preventive measures.
10. Is there a classical Ayurvedic verse supporting cancer prevention or cure?
Yes. “Vajikarana Rasayana Sevanaat Balya Varna Swara Prabha” (Charaka Samhita, Chikitsa Sthana 1.1) – Rasayana therapy enhances strength, complexion, immunity, and resistance against disease, including growth disorders.
References
Note: Every reference listed here has been carefully selected for accuracy, clinical relevance, and traceability. Ayurvedic formulations are cited directly from classical medical texts (Charaka Samhita, Sushruta Samhita, Bhavaprakasha, etc.) along with specific verse numbers and chapters. All modern scientific studies are provided with active hyperlinks in APA format. This dual validation—classical and contemporary—ensures the highest integrity of information for patients, practitioners, and researchers.
If you find any reference missing or wish to request full-text access for a particular citation, you may contact the author directly. Our goal is to maintain complete transparency and academic rigor.
[1] Charaka Samhita, Chikitsa Sthana, Chapter 1, Verse 7–12. Commentary by Chakrapani Datta. (2018). Chaukhamba Orientalia, Varanasi. Classical reference to Rasayana chikitsa for systemic rejuvenation and prevention of chronic diseases.
[2] Sushruta Samhita, Chikitsa Sthana, Chapter 11, Verse 3–6. (2017). Chaukhamba Sanskrit Pratishthan, Delhi. Details the role of Raktamokshana and purification in pathological growths.
[3] Bhavaprakasha Nighantu, Haritakyadi Varga, Shloka 42–48. (2016). Chaukhamba Bharati Academy, Varanasi. Describes the properties of Amalaki in detoxification and immunity enhancement.
[4] Rasatarangini, Taranga 15, Verse 29–35. (2015). Motilal Banarsidass Publishers, Delhi. Classical formulation methods for Swarna Bhasma and its Rasayana properties.
[5] Sharma, V., & Singh, G. (2021). Pharmacognostic evaluation and therapeutic potential of Withania somnifera in cancer care. Journal of Ethnopharmacology, 281, 114521. https://doi.org/10.1016/j.jep.2021.114521
[6] Kaur, P., et al. (2020). Shatavari (Asparagus racemosus) and its role in female reproductive health: A review. Phytotherapy Research, 34(6), 1243–1256. https://doi.org/10.1002/ptr.6604
[7] Singh, R. H., et al. (2019). Clinical evaluation of Kanchnar Guggulu in benign and malignant growths. AYU Journal, 40(3), 160–168. https://doi.org/10.4103/ayu.AYU_170_19
[8] Rasendra Chintamani, Chapter 6, Verse 10–14. (2015). Chaukhamba Surbharati Prakashan, Varanasi. Describes Heerak Bhasma in chronic diseases including abnormal cell proliferation.
[9] Gupta, S. C., et al. (2019). Antiproliferative and pro-apoptotic properties of Kanchnar Guggulu: Evidence from in vitro studies. Integrative Cancer Therapies, 18, 153473541988229. https://doi.org/10.1177/1534735419882292
[10] Sharma, R., et al. (2020). Abhrak Bhasma as a nano-herbo-mineral formulation: Characterization and therapeutic applications. Journal of Ayurveda and Integrative Medicine, 11(3), 300–308. https://doi.org/10.1016/j.jaim.2018.12.002
[11] Lord, C. J., & Ashworth, A. (2017). PARP inhibitors: Synthetic lethality in the clinic. Science, 355(6330), 1152–1158. https://doi.org/10.1126/science.aam7344
[12] Moore, K., et al. (2018). Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. The New England Journal of Medicine, 379(26), 2495–2505. https://doi.org/10.1056/NEJMoa1810858
[13] Pujade-Lauraine, E., et al. (2017). Olaparib tablets as maintenance therapy in platinum-sensitive relapsed ovarian cancer. The Lancet Oncology, 18(9), 1274–1284. https://doi.org/10.1016/S1470-2045(17)30469-2
[14] Konstantinopoulos, P. A., et al. (2020). Immunotherapy in ovarian cancer: PD-1/PD-L1 inhibition and beyond. Clinical Cancer Research, 26(22), 5939–5945. https://doi.org/10.1158/1078-0432.CCR-20-0941
[15] Morgan, R. J., et al. (2021). Platinum-based chemotherapy in BRCA-mutated ovarian cancer: Clinical perspectives. Gynecologic Oncology, 161(2), 623–630. https://doi.org/10.1016/j.ygyno.2021.02.004
[16] Dutta, R., & Sharma, D. (2021). Ayurvedic Rasayana and Satvavajaya Chikitsa in oncology care: A clinical review. Journal of Ayurveda Case Reports, 4(1), 45–52. https://doi.org/10.4103/jacr.jacr_8_21
[17] Singh, R., et al. (2020). Yoga and Pranayama in cancer survivorship: A systematic review. Complementary Therapies in Medicine, 52, 102502. https://doi.org/10.1016/j.ctim.2020.102502
[18] Kumar, A., et al. (2018). Nanomedicine perspectives of Ayurvedic Bhasma formulations in cancer therapy. Current Pharmaceutical Design, 24(30), 3570–3581. https://doi.org/10.2174/1381612824666180925120744
[19] Chauhan, A., et al. (2019). Amalaki Rasayana as a bio-enhancer and chemopreventive agent. Journal of Herbal Medicine, 15, 100225. https://doi.org/10.1016/j.hermed.2018.12.003
[20] Gupta, Y., & Tillu, G. (2020). Evidence synthesis on Ayurvedic interventions in oncology. Indian Journal of Traditional Knowledge, 19(2), 314–327. https://doi.org/10.56042/ijtk.v19i2.41554
[21] Sharma, S., et al. (2021). Potential role of phytoconstituents in reversing chemoresistance. Phytomedicine, 87, 153580. https://doi.org/10.1016/j.phymed.2021.153580
[22] Singh, M., et al. (2022). Ayurvedic mineral formulations in integrative oncology: Safety and efficacy review. Integrative Medicine Research, 11(4), 100910. https://doi.org/10.1016/j.imr.2022.100910