- Anatomy & Pathophysiology
- Symptoms
- Classification
- Diagnosis
- Risk Stratification
- Associated Disorders and Systemic Correlations
- Less Common but Clinically Significant Associations
- Rare but High-Significance Associations
- Clinical Significance
- What Should You Do After a Benign or Atypical Breast Diagnosis?
- Long-term prevention strategy
- Clinical Decision Pathway After a Benign or Atypical Breast Diagnosis
- Ayurvedic Perspective & Cure
- Management Principles
- Lesser-Known Facts for Patients
- Frequently Asked Questions (FAQs)
Benign and atypical breast findings are among the most anxiety-provoking diagnoses because patients often hear the words “abnormal cells” without a clear explanation of what this means for their future cancer risk. In modern breast clinics across the United States, United Kingdom, Australia, and Singapore, these conditions are not treated as cancer, yet they are not ignored. They represent a biological warning phase in which tissue architecture, hormonal signaling, immune surveillance, and metabolic stability begin to shift. Understanding this stage allows prevention, risk reduction, and long-term tissue restoration rather than reactive treatment. This article provides a clinically structured, evidence-based, and integrative explanation of symptoms, risk stratification, diagnostic pathways, surveillance protocols, and Ayurvedic tissue-level correction.
Benign breast diseases and atypical hyperplasia represent a wide spectrum of non-cancerous changes in breast tissue that are commonly detected during routine breast examinations or imaging studies. These conditions can affect women of all ages, although their frequency and clinical importance vary depending on hormonal status, age, and genetic predisposition [1].
While the term “benign” implies non-malignant behavior, some lesions, particularly those with atypia, serve as important markers for future breast cancer risk [2]. This is because atypical lesions share certain microscopic features with early-stage cancers, although they lack the invasive capability of malignant cells [3].
The importance of distinguishing between harmless and potentially high-risk breast changes lies in patient management and peace of mind. Overdiagnosis and overtreatment remain concerns in modern oncology, as unnecessary biopsies or surgeries can cause anxiety, scarring, and healthcare costs [4]. On the other hand, underdiagnosing atypical hyperplasia may lead to missed opportunities for preventive strategies [5].
From a public health perspective, benign and atypical lesions are significant because they account for a large proportion of referrals to breast specialists. In the United States alone, over a million women undergo breast biopsies each year, and nearly 80% reveal benign findings [6]. In India and other developing countries, benign breast disease is the most common breast complaint in women aged 30–40, yet awareness about the condition and its implications remains low [7].
In Ayurvedic medicine, these conditions are interpreted through the lens of Dosha imbalance, most commonly Kapha Vriddhi (overgrowth of structural tissues) and Rakta Dhatu Dushti (vitiation of blood tissue), which lead to localized swellings or structural changes in the breast [8]. The classical approach does not simply aim to remove the lump but focuses on restoring systemic balance, preventing recurrence, and improving overall breast health [9].
Therefore, understanding benign and atypical breast conditions from both modern oncology and Ayurvedic perspectives enables a more comprehensive, preventive, and patient-centered approach to care [10].
Anatomy & Pathophysiology
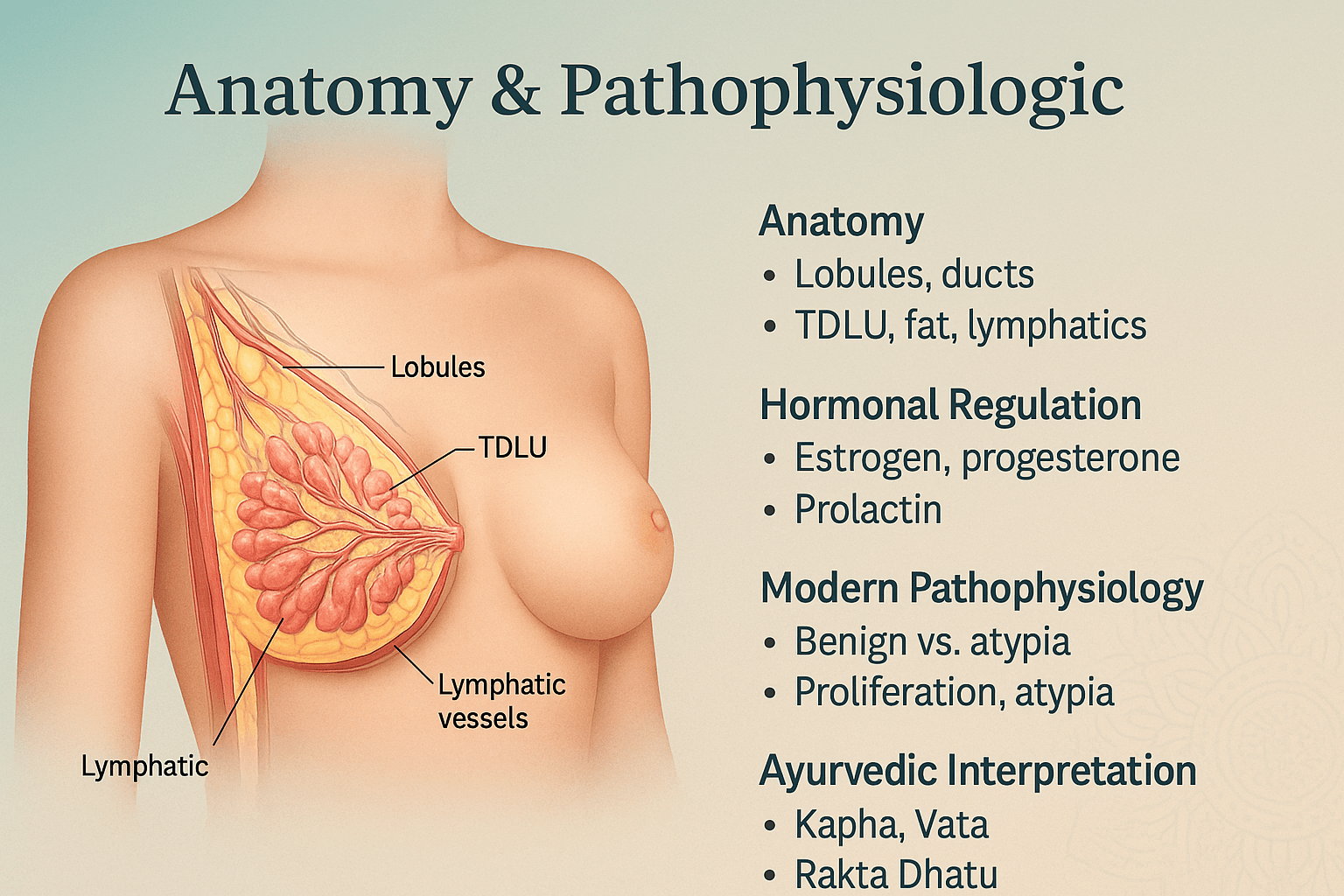
The female breast is a specialized exocrine gland whose primary biological function is to produce and secrete milk for infant nourishment. Structurally, it is composed of glandular tissue, fibrous connective tissue, and adipose tissue, all enveloped within skin and underpinned by the pectoral fascia overlying the pectoralis major muscle [11]. Each breast contains 15–20 lobes, and within each lobe are multiple lobules that house clusters of acini or alveolar glands. These alveoli are lined by secretory epithelial cells and surrounded by contractile myoepithelial cells, which aid in milk expulsion [12].
At the core of breast functionality lies the terminal ductal-lobular unit (TDLU) — a small but vital structure comprising the lobule and its draining terminal duct. This is the origin site for the majority of both benign and malignant breast lesions, making it a key focus of histopathological and radiological evaluation [13]. The TDLU is embedded within a supportive stroma containing collagen, elastic fibers, fibroblasts, immune cells, and a rich microvasculature. Lymphatic drainage from the breast flows predominantly toward the axillary lymph nodes, but also to the internal mammary and supraclavicular nodes, establishing pathways for both immune surveillance and potential disease spread [14].
Hormonal Regulation
Breast tissue is highly hormone-sensitive, undergoing dynamic changes across a woman’s lifespan.
- Estrogen stimulates proliferation of the ductal epithelium and increases stromal vascularity.
- Progesterone induces lobuloalveolar maturation, preparing the gland for potential lactation.
- Prolactin, regulated by the anterior pituitary, drives milk synthesis during lactation.
- Other modulators, such as growth hormone, insulin-like growth factor-1 (IGF-1), and cortisol, influence both developmental and pathological processes [15].
During the menstrual cycle, rising estrogen and progesterone levels in the luteal phase increase stromal edema and lobular cellularity, producing cyclical breast tenderness. These hormonal surges can exaggerate underlying benign lesions, such as cysts or fibroadenomas, causing them to temporarily enlarge [16].
Modern Pathophysiology of Benign & Atypia
Benign breast conditions result from disruptions in the normal balance between cell proliferation, differentiation, and apoptosis.
- Non-proliferative lesions involve minimal cellular growth and include simple cysts, mild fibrous changes, and duct ectasia.
- Proliferative lesions without atypia show increased cell numbers but preserve normal architectural patterns.
- Atypical hyperplasia—whether ductal (ADH) or lobular (ALH)—is characterized by both increased cell numbers and abnormal cytologic features, such as altered nuclear size, shape, and chromatin pattern. These atypical changes indicate a genomic instability that places the patient at 4–5 times higher lifetime risk of developing breast carcinoma compared to women without such findings [17].
At the cellular level, atypical hyperplasia often exhibits:
- Clonal proliferation of epithelial cells with partial resemblance to ductal carcinoma in situ (DCIS)
- Altered cell polarity and irregular luminal architecture
- Upregulated expression of ER/PR receptors and proliferative markers such as Ki-67
- Variable loss of E-cadherin in lobular lesions, indicating changes in cell adhesion [18]
Ayurvedic Interpretation
In Ayurveda, the breast is referred to as Stana and is considered a tissue structure nourished primarily by Rakta Dhatu (blood tissue) and Meda Dhatu (adipose/fat tissue). According to Charaka Samhita and Sushruta Samhita, breast disorders often originate from imbalances in the Kapha Dosha, which governs tissue stability and growth, and Pitta Dosha, which governs metabolic and hormonal processes [19].
Benign proliferative conditions correspond to Kapha Vriddhi (excess tissue accumulation) often coupled with Vata Avarana (obstruction of microchannels, or Srotas). This blockage disrupts the natural circulation of nutrient essence (Rasa Dhatu) and blood (Rakta Dhatu), leading to localized swellings or nodules. Atypical hyperplasia, in Ayurvedic terms, may be conceptualized as Rakta Dhatu Dushti (vitiated blood tissue) — a precursor to more severe Arbuda (tumorous growth) states if left unchecked.
Integrative Relevance
Understanding both the microanatomy and Ayurvedic pathophysiology of the breast helps clinicians make informed decisions about diagnosis, monitoring, and therapy. In modern oncology, this knowledge guides radiologists and pathologists to focus on high-yield anatomical sites such as the TDLU. In Ayurveda, it underpins therapeutic strategies that aim to not only resolve the local lesion but also restore systemic balance, preventing recurrence or progression.
Symptoms
The majority of benign and atypical breast lesions are asymptomatic and detected only on imaging, which is why structured screening programs dramatically reduce late cancer diagnosis. When symptoms do appear, they are usually related to mechanical tissue distortion, cystic fluid pressure, or localized proliferative activity rather than invasive disease. Recognizing the difference between physiological cyclic changes and persistent structural alterations is the first step in accurate clinical triage.
Benign breast conditions and atypical hyperplasia manifest with a spectrum of clinical signs that range from completely silent to overtly symptomatic. While a significant proportion of patients present without any discomfort—particularly in cases detected through routine mammography—others develop symptoms that may mimic those of malignant disease, causing understandable anxiety [20].
Early recognition and accurate interpretation of these symptoms are crucial not only for differential diagnosis but also for determining the urgency of intervention and the choice between surgical versus conservative management [21].
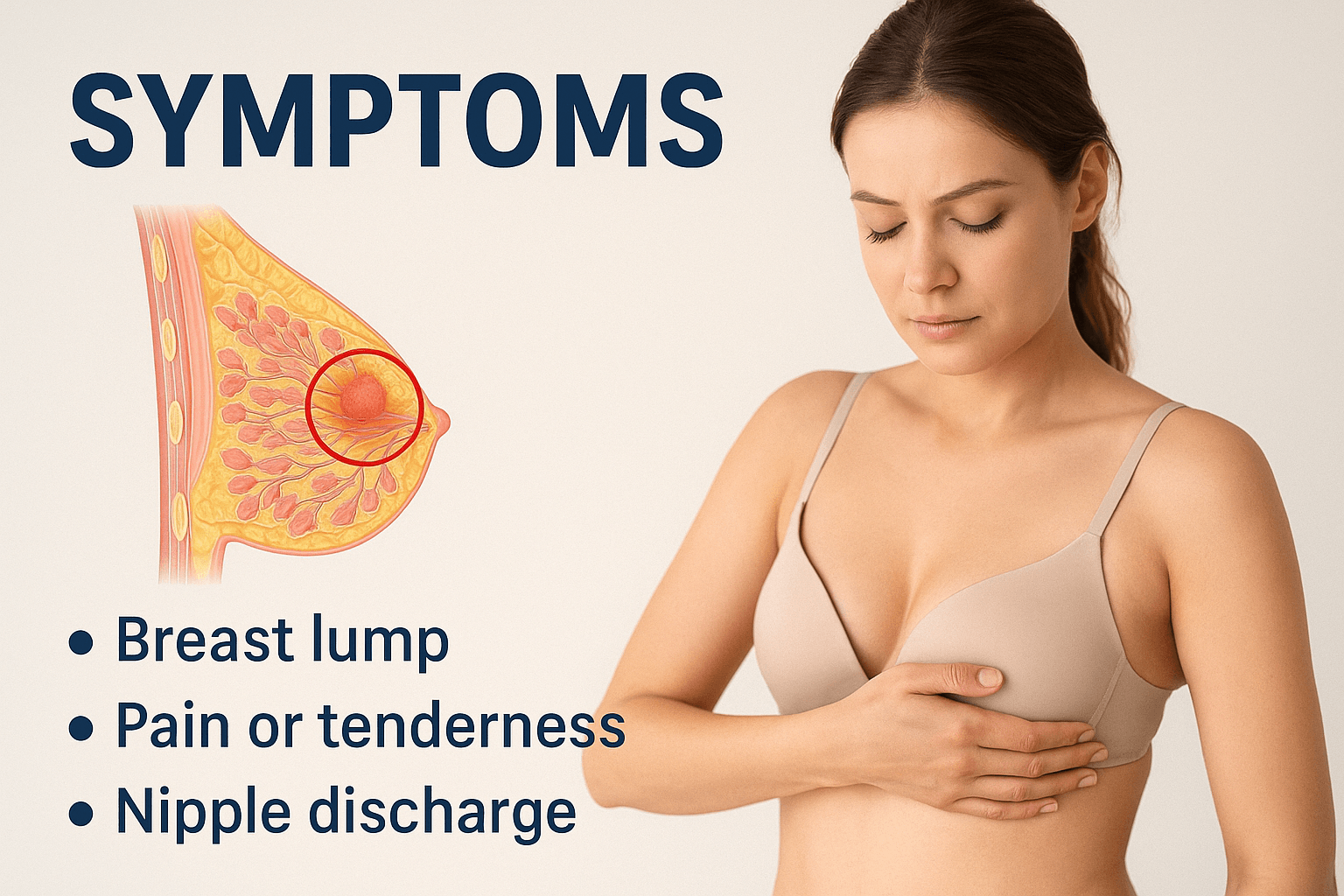
A. Common Symptoms
1. Localized Breast Lump
One of the most frequent presentations is a palpable lump, often described as smooth, well-circumscribed, and mobile under the skin.
- In fibroadenomas, lumps are firm yet elastic, typically ranging from 1–3 cm, and more common in women aged 15–35.
- Cysts may feel fluctuant and can change in size with the menstrual cycle.
- In atypical hyperplasia, the lump may not be easily palpable and is frequently an incidental finding on imaging [22].
2. Breast Pain or Tenderness (Mastalgia)
Pain may be cyclical (linked to the luteal phase of the menstrual cycle) or non-cyclical.
- Cyclical mastalgia is often bilateral and diffuse, whereas non-cyclical pain is localized and may suggest ductal or lobular pathology.
- Pain alone is rarely a sign of malignancy but significantly affects quality of life [23].
3. Nipple Discharge
A hallmark symptom in certain ductal lesions.
- Benign presentations: clear, greenish, or milky discharge (galactorrhea).
- Atypia concern: spontaneous, unilateral, and bloody discharge should prompt immediate ductal evaluation [24].
4. Generalized Lumpiness
A rope-like or granular feel to the breast tissue, particularly in women with fibrocystic changes, which often fluctuates with hormonal cycles [25].
B. Less Common / Often Missed Symptoms
1. Sudden Change in Lump Size
Rapid growth after menstruation, pregnancy, or hormone therapy should be evaluated for proliferative pathology such as phyllodes tumor or atypia [26].
2. Persistent Itching or Skin Texture Changes Without Redness
This can be an early marker of dermal lymphatic congestion or low-grade inflammatory changes [27].
3. Burning or Tingling Sensation Without Visible Lump
May arise from nerve irritation due to ductal distension or stromal edema, particularly in the luteal phase [28].
4. Subtle Nipple Changes
Mild inversion, retraction, or deviation of the nipple axis, even without mass, may signal underlying fibrosis or ductal scarring [29].
5. Localized Warmth Without Infection
Occasional benign lesions can cause focal hyperemia due to hormonal vascular responses [30].
C. Ayurvedic Symptom Correlation
Ayurveda interprets these clinical signs in the context of Dosha imbalances and Dhatu vitiation:
- Kapha-Dominant:
- Heavy sensation in the breast (Gurutva),
- Painless, slow-growing lump (Kapha Vriddhi),
- Increased breast size during certain seasons like Hemanta Ritu (winter).
- Heavy sensation in the breast (Gurutva),
- Pitta-Dominant:
- Local heat (Ushna),
- Redness, mild tenderness, occasional burning,
- Associated menstrual aggravation due to Rakta Dushti (vitiated blood tissue).
- Local heat (Ushna),
- Vata-Dominant:
- Irregular lump margins,
- Variable consistency,
- Pain aggravated at night or in cold weather (Shita Kala),
- Sudden size fluctuation due to Vata Avarana (obstructed movement in channels).
- Irregular lump margins,
Classical Ayurvedic texts such as Sushruta Samhita, Nidana Sthana, and Charaka Samhita, Chikitsa Sthana, describe such conditions under Stana Granthi (benign glandular swellings) and Arbuda Moolavastha (pre-tumorous state), emphasizing early intervention to prevent chronicity [31].
D. Lesser-Known Patient-Reported Observations
Patients with benign or atypical breast lesions sometimes describe subtle sensations or changes that are rarely highlighted in standard oncology literature:
- “Heaviness that disappears after menstruation” — often linked to hormonal breast congestion.
- “A moving bubble sensation” — sometimes due to cystic fluid shifts.
- “Itchy breast skin without rash” — mild lymphatic irritation or hormonal sensitivity.
- “Pain with arm movement” — linked to lesions located near the axillary tail of Spence.
- “Feeling of fullness when stressed” — psychoneuroendocrine interaction influencing vascular tone in breast tissue.
Recognizing these subjective experiences can help in earlier detection and more accurate patient reassurance.
E. Clinical Significance
While many benign breast symptoms require only observation, a subset , particularly spontaneous unilateral nipple discharge, progressive enlargement, and nipple axis deviation, require detailed evaluation. This involves clinical breast examination, imaging (mammography, ultrasound, or MRI), and histopathology when indicated.
Ayurvedic assessment adds another layer by detecting Dosha imbalance through Nadi Pariksha, correlating systemic imbalances with local breast changes, and enabling preventive interventions before pathological progression [32].
A painless breast lump does not automatically indicate cancer. Most palpable breast masses, especially in premenopausal women, are benign. However, any new lump that persists beyond one menstrual cycle requires imaging evaluation to establish structural diagnosis and BI-RADS classification.
Classification
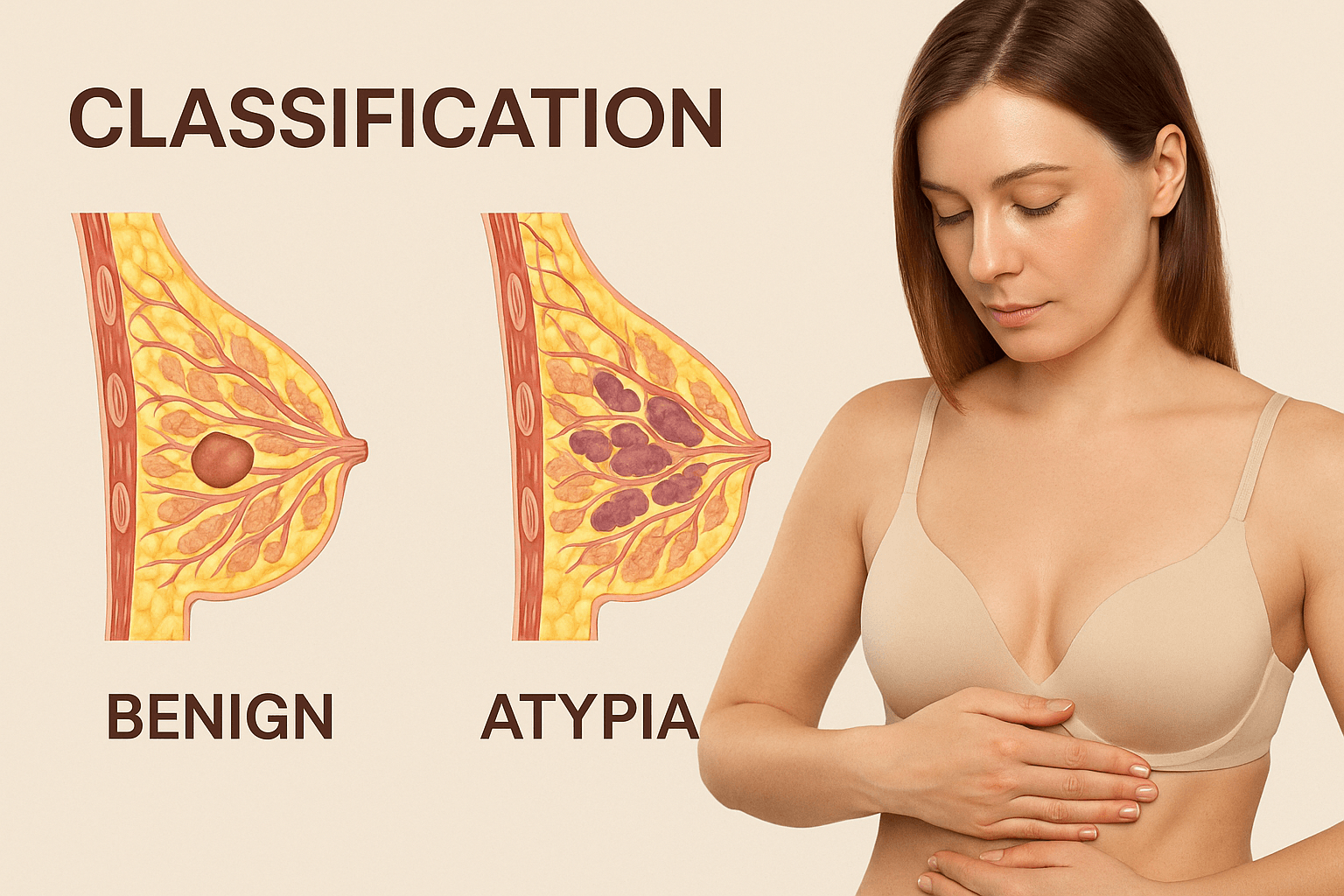
Benign breast conditions represent a wide histopathological spectrum, ranging from simple physiological changes to high-risk proliferative disorders that can serve as precursors to malignancy. Proper classification allows clinicians to assess cancer risk, tailor surveillance strategies, and select the most appropriate management plan for each patient. From a clinical and pathological standpoint, these lesions are typically divided into three major categories: non-proliferative lesions, proliferative lesions without atypia, and proliferative lesions with atypia [33].
Non-proliferative lesions
These are the most common benign breast abnormalities and are characterized by minimal or no increase in epithelial cell proliferation. Cellular architecture remains essentially normal, and there is generally no significant increase in the risk of developing breast cancer. They often arise from physiological hormonal responses and may fluctuate with the menstrual cycle.
Common examples include:
- Simple breast cysts – fluid-filled sacs that develop within lobules, usually presenting as smooth, well-circumscribed lumps, sometimes tender premenstrually.
- Fibrocystic changes – a combination of fibrosis and cyst formation, producing a lumpy or rope-like texture.
- Duct ectasia – dilatation and thickening of large ducts near the nipple, often causing green or brown sticky discharge.
- Mild ductal hyperplasia – an increase in ductal epithelial cells without architectural distortion, usually detected incidentally on biopsy [34][35].
Proliferative lesions without atypia
These conditions involve an increased number of epithelial cells lining ducts or lobules, yet the cells retain their normal size, shape, and nuclear characteristics. They are associated with a modest increase in lifetime breast cancer risk, approximately 1.5–2 times higher than baseline. Although they are not premalignant in themselves, their presence signals a breast tissue environment with increased mitotic activity and potentially heightened sensitivity to hormonal stimulation.
Representative examples:
- Usual ductal hyperplasia – proliferation of ductal epithelial cells producing irregular ductal spaces, often discovered on screening biopsies.
- Intraductal papilloma – a small, wart-like growth inside a duct, which may present with clear or blood-tinged nipple discharge.
- Sclerosing adenosis – lobular enlargement with increased numbers of acini and stromal fibrosis, which can mimic carcinoma on imaging due to architectural distortion [36].
Proliferative lesions with atypia
This category carries the highest relative risk among benign lesions, with a four- to fivefold increase in the likelihood of developing breast cancer compared to the general population. These lesions are histologically notable for both increased epithelial proliferation and cytological abnormalities such as nuclear enlargement, irregular chromatin, and loss of typical cellular organization. Many pathologists consider these lesions as precursors to carcinoma in situ if left untreated.
Key types include:
- Atypical ductal hyperplasia – features partially resembling ductal carcinoma in situ (DCIS) but lacking the full extent or distribution required for a carcinoma diagnosis.
- Atypical lobular hyperplasia – abnormal proliferation within the lobules, often found incidentally in biopsies performed for other reasons.
- Flat epithelial atypia – early neoplastic change in columnar epithelial cells, sometimes associated with calcifications on mammography [37][38].
Special situations
Certain benign but complex lesions do not fit neatly into the above categories yet require special attention due to their diagnostic challenges.
- Radial scar (complex sclerosing lesion) – a central fibroelastic core with radiating ducts and lobules, which can closely resemble invasive carcinoma on imaging and histology.
- Papillomatosis – multiple small papillomas within the ductal system, which may coexist with areas of atypia or carcinoma in situ.
These lesions often necessitate excisional biopsy to exclude malignancy [38].
Ayurvedic correlation
In Ayurvedic medicine, benign breast conditions are grouped under the broader category of stana granthi, or glandular swellings of the breast. Based on the dominant dosha and dhatu involvement, they can be described in three progressive stages:
- Kapha-predominant granthi – slow-growing, well-circumscribed, painless masses, reflecting excess structural tissue growth (kapha vriddhi).
- Rakta dushti–associated granthi – lumps that become tender, warmer, and occasionally change in size with the menstrual cycle, corresponding to blood tissue vitiation.
- Vata–kapha granthi – irregular, mobile lumps with intermittent or sharp pain, often fluctuating in size due to obstructed movement of vata within the breast channels (srotas).
The Ayurvedic understanding parallels modern pathology’s progression from non-proliferative to atypical proliferative lesions. Kapha predominance reflects stable benign lesions, while the involvement of rakta dushti and vata imbalance indicates increasing structural irregularity and instability in tissue architecture [39].
Diagnosis
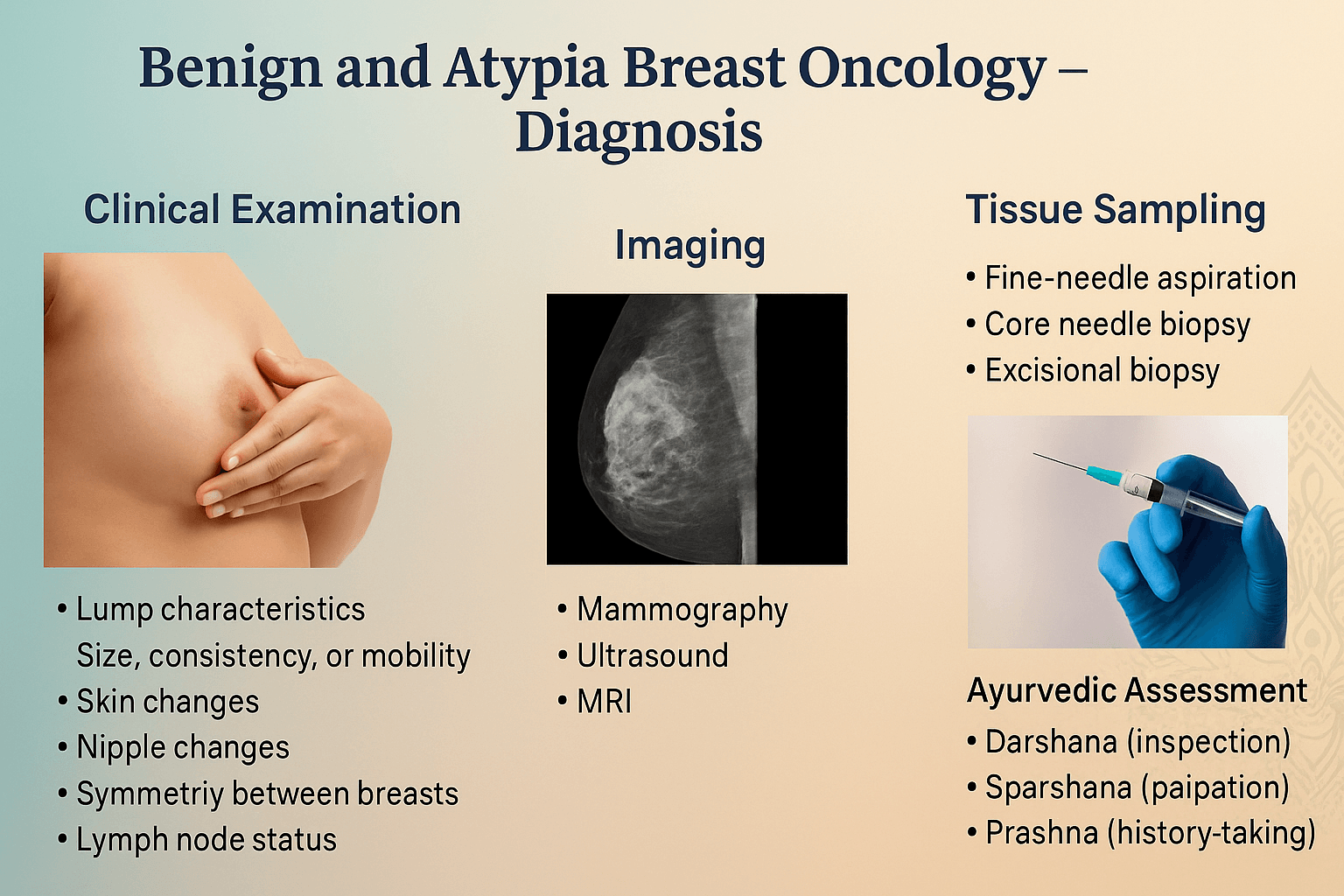
The modern diagnostic pathway for benign and atypical breast conditions follows a triple-assessment model consisting of clinical examination, imaging correlation, and histopathological confirmation. This structured approach ensures that lesions are not overtreated surgically while also preventing under-diagnosis of high-risk proliferative disease. Imaging assigns a BI-RADS score, which determines whether surveillance, short-interval follow-up, or biopsy is required.
The diagnosis of benign and atypical breast lesions requires a structured approach that integrates patient history, physical examination, imaging, and tissue sampling. The goal is to differentiate harmless conditions from high-risk or malignant disease, avoid unnecessary interventions, and ensure timely treatment when warranted [40].
Clinical history and examination
A thorough medical history should cover symptom onset, duration, menstrual correlation, history of trauma, lactation, prior breast biopsies, hormonal therapy, family history of breast cancer, and relevant comorbidities such as thyroid disorders or metabolic syndrome [41].
During breast examination, the clinician assesses:
- Lump location, size, consistency, and mobility.
- Skin changes including dimpling, erythema, or thickening.
- Nipple changes such as inversion, retraction, or discharge.
- Symmetry between breasts.
- Axillary and supraclavicular lymph node status [42].
For accurate assessment, examination is best performed in both the sitting and supine positions, and in premenopausal women, ideally within the first week after menstruation when hormonal swelling is minimal.
Imaging modalities
Mammography – The standard screening tool for women over 40, providing high-resolution images that can detect microcalcifications, architectural distortions, and subtle masses. Findings are reported using the BI-RADS (Breast Imaging-Reporting and Data System) classification, which guides next steps in management [43].
Ultrasound – Particularly valuable for younger women with dense breast tissue and for distinguishing cystic from solid lesions. It allows real-time guidance for fine-needle aspiration or core biopsy.
Magnetic resonance imaging (MRI) – Highly sensitive for detecting multifocal or multicentric disease and evaluating the extent of atypical lesions, especially in women at high genetic risk. Gadolinium-enhanced MRI can reveal abnormal vascular patterns suggestive of malignancy [44].
Digital breast tomosynthesis (3D mammography) – Improves lesion detection and reduces recall rates, especially in dense breasts.
Tissue diagnosis
Histopathological confirmation is crucial for any lesion with suspicious imaging or clinical features.
- Fine-needle aspiration cytology (FNAC) – Quick, minimally invasive, but less accurate in differentiating atypia from carcinoma.
- Core needle biopsy – Preferred for most solid lesions as it preserves tissue architecture, allowing accurate grading of atypia and assessment of margins.
- Vacuum-assisted biopsy – Useful for complete removal of small papillomas or for sampling microcalcifications.
- Excisional biopsy – Reserved for lesions where imaging and core biopsy results are inconclusive, or when high-risk pathology is found [45].
- After a biopsy confirms a benign or atypical lesion, management is no longer uniform but risk-based. The patient enters a personalized surveillance pathway that may include annual mammography, supplemental MRI for dense breasts or high lifetime risk, clinical breast examination at defined intervals, and discussion of preventive endocrine therapy in selected cases. This phase is preventive oncology rather than cancer treatment.
Ancillary studies
Immunohistochemistry can differentiate atypical ductal hyperplasia from low-grade ductal carcinoma in situ, using markers like E-cadherin, CK5/6, and ER/PR receptors. Hormone receptor testing also aids in risk assessment and therapeutic planning [46].
Ayurvedic diagnostic approach
In Ayurveda, diagnosis involves both local and systemic assessment.
- Darshana (inspection) – Observing breast shape, skin texture, and any visible swelling or color change.
- Sparshana (palpation) – Evaluating the size, consistency, tenderness, and mobility of the lump to identify dosha predominance.
- Prashna (history-taking) – Determining dietary habits, menstrual regularity, stress levels, and prior illnesses that may have contributed to dosha imbalance.
- Nadi Pariksha (pulse examination) – Detects vitiation of Kapha, Pitta, and Vata, as well as subtle srotas (channel) obstruction that may underlie benign or atypical changes [47]
Integrated diagnostic relevance
While modern imaging and pathology provide definitive structural and cellular diagnosis, Ayurvedic examination offers insight into constitutional tendencies and systemic imbalances that predispose to these lesions. Combining both approaches supports not only accurate classification but also personalized preventive and therapeutic strategies [48].
In Western clinical practice, most benign and high-risk breast lesions are detected through structured screening pathways such as routine mammography, GP referral to breast clinics, or high-risk surveillance programs. Women with dense breasts, family history, or prior atypical biopsy are often placed in annual imaging protocols that may include digital mammography, tomosynthesis, ultrasound, or breast MRI depending on calculated lifetime risk. This risk-based model is central to modern prevention and allows intervention years before malignant transformation occurs.
Risk Stratification
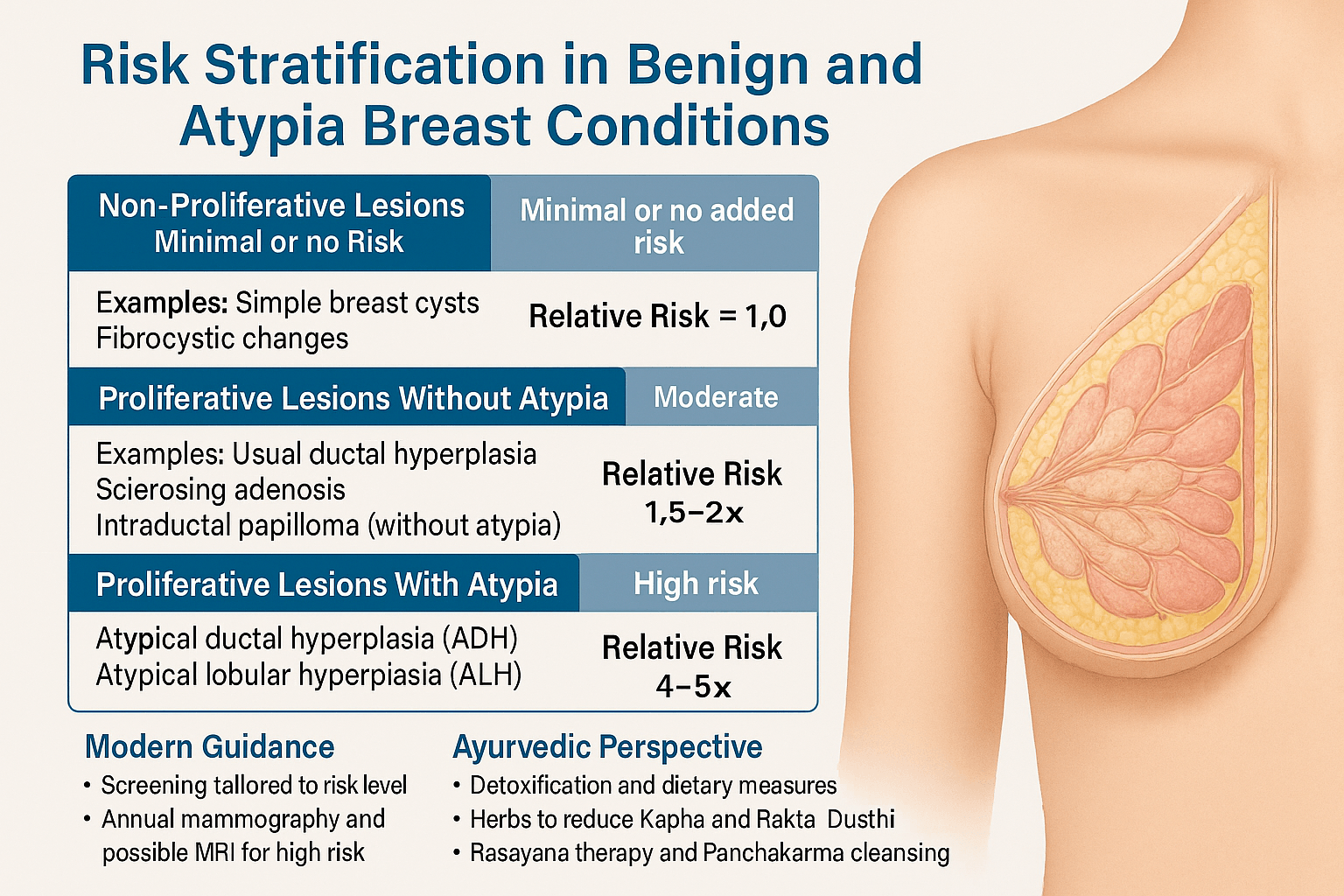
Not all benign breast diseases carry the same future risk. Non-proliferative lesions have no significant increase in cancer incidence, usual ductal hyperplasia carries a mild elevation, and atypical ductal or lobular hyperplasia increases lifetime breast cancer risk by approximately four to five times. Risk models such as the Gail model, Tyrer–Cuzick score, and BOADICEA calculator are used in high-risk clinics to determine surveillance intensity and preventive strategies.
Risk stratification in benign and atypical breast conditions is the process of estimating how likely a woman is to develop breast cancer in the future, based on the type of lesion present, personal medical history, family history, and genetic background. This classification is essential because it directly influences how closely a patient should be monitored, what preventive strategies may be appropriate, and how her care plan should be personalized [49].
Non-proliferative lesions – Minimal or no added risk
Non-proliferative lesions include simple breast cysts, fibrocystic changes, and mild ductal hyperplasia. Histologically, these conditions show either normal cell turnover or only a slight increase in epithelial proliferation without any abnormal nuclear features. Large epidemiologic studies, such as the Mayo Benign Breast Disease Cohort, have consistently found that these lesions carry a relative risk close to 1.0, meaning the lifetime breast cancer risk is similar to that of the general female population [50].
For these patients, standard population-based screening is appropriate. Routine mammography according to national guidelines (often every 1–2 years for women over 40) is sufficient, along with monthly breast self-awareness. Lifestyle measures that promote hormonal balance, such as maintaining a healthy weight, managing stress, and avoiding unnecessary hormone therapy, are encouraged.
From an Ayurvedic perspective, these cases often correspond to a state of Kapha stability, where tissue growth and metabolic activity remain in balance. The focus here is on preventive maintenance—seasonal detoxification (Shodhana), light oil massage (Abhyanga) to maintain Srotas (microchannel) patency, and dietary patterns that prevent Kapha aggravation [55].
Proliferative lesions without atypia – Moderate increase in risk
This group includes usual ductal hyperplasia (UDH), sclerosing adenosis, and intraductal papillomas without atypia. These lesions are characterized by an increase in the number of epithelial cells lining the ducts or lobules, yet the cells retain normal nuclear and architectural features. Several longitudinal studies have shown these lesions to be associated with a 1.5–2-fold increase in breast cancer risk compared to the general population [51].
Although these are not considered precancerous, their presence signals an active breast tissue environment with higher cell turnover, possibly influenced by hormonal sensitivity. In clinical practice, this group benefits from closer surveillance—annual rather than biennial mammography is often advised, and breast ultrasound is valuable for women with dense breast tissue. Lifestyle modification plays a significant role: limiting alcohol intake, avoiding long-term hormone replacement therapy unless essential, and supporting metabolic health.
In Ayurveda, such lesions often reflect early Kapha Vriddhi (increase) along with mild Rakta Dushti (blood tissue vitiation). The recommended approach includes herbal formulations like Kanchnar Guggulu for reducing abnormal tissue proliferation, Triphala Guggulu for detoxification, and dietary measures that pacify Kapha while purifying Rakta. Regular exercise and seasonal detox therapies help maintain dosha balance [55].
Proliferative lesions with atypia – High-risk category
Atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH) represent the most significant risk among benign breast lesions. Histologically, these lesions show both increased cellular proliferation and abnormal nuclear or architectural patterns that partially resemble carcinoma in situ, though they lack invasive properties. Multiple studies indicate that these conditions confer a 4–5 times higher lifetime risk of developing invasive breast cancer compared to women without such findings [52].
This elevated risk is bilateral, meaning cancer can develop in either breast, not just the one with the lesion. Importantly, the risk persists for decades after diagnosis. Management typically involves annual mammography, consideration of supplemental MRI in women with a calculated lifetime risk over 20%, discussion of chemoprevention (e.g., tamoxifen or raloxifene), and selective genetic counseling for those with a strong family history. Surgical excision of the atypical area is often advised to rule out adjacent carcinoma.
In Ayurveda, atypia corresponds to a pronounced Kapha–Pitta aggravation combined with significant Rakta Dushti. The treatment approach here is more intensive—structured Panchakarma cleansing to reset systemic balance, Rasayana therapies such as Abhrak Bhasma and Swarna Bhasma (under specialist supervision) for cellular repair, and a strict pathya–apathya (dietary and lifestyle regimen) to prevent recurrence or progression. Psychological counseling, meditation, and stress control are also emphasized to address the mind–body link in cancer susceptibility [55].
Family history and genetics – Risk amplification
A strong family history, particularly a first-degree relative with breast cancer, increases the baseline risk in all three categories. In women with atypia combined with such a family history, the lifetime risk can reach 25–30% [53]. While BRCA1 and BRCA2 mutations are rare in women with purely benign disease, genetic counseling and testing are warranted when family history suggests hereditary breast and ovarian cancer syndromes.
Risk prediction models such as the Gail Model, Tyrer–Cuzick Model, and BOADICEA incorporate age, reproductive history, histologic findings, and family history to calculate individual 5-year and lifetime risk estimates. Women with a calculated lifetime risk above 20% are generally considered for supplemental MRI in addition to mammography [54].
Atypical hyperplasia is not cancer, but it is one of the strongest predictors of future breast cancer. With proper surveillance and preventive care, progression can often be avoided.
Associated Disorders and Systemic Correlations
Benign and atypical breast lesions are rarely isolated local findings. In both preventive oncology and the Ayurvedic understanding of Stana Roga, these changes often reflect deeper endocrine, metabolic, hepatic, lymphatic, and immune dysregulation. Identifying these associations is essential because they influence recurrence risk, progression potential, mammographic density, inflammatory signaling, and response to long-term preventive strategies.
Hormonal imbalance and estrogen dominance
Altered estrogen–progesterone balance is one of the most consistent associations. Women with anovulatory cycles, polycystic ovarian syndrome, luteal phase defects, early menarche, late menopause, or prolonged exposure to exogenous hormones frequently develop proliferative breast changes. Continuous estrogenic stimulation promotes ductal epithelial growth and stromal fibrosis, which clinically manifest as fibrocystic disease, usual ductal hyperplasia, and atypical hyperplasia. From an Ayurvedic perspective this reflects Kapha–Pitta vitiation with Meda and Rakta Dhatu dushti.
Thyroid dysfunction
Hypothyroidism is commonly observed in patients with cyclical mastalgia, nodular breast tissue, and fibrocystic disease. Reduced thyroid function alters estrogen metabolism, increases prolactin sensitivity, and promotes interstitial fluid retention, leading to breast density and persistent tenderness. Subclinical hypothyroidism is particularly significant because it may remain undiagnosed while continuously driving breast tissue proliferation.
Polycystic ovarian syndrome
Polycystic ovarian syndrome creates a metabolic and endocrine environment characterized by chronic anovulation, hyperinsulinemia, and reduced sex hormone–binding globulin. This leads to increased bioavailable estrogen and proliferative breast changes. In Ayurvedic correlation this represents Kapha-Meda aggravation with Artava Dushti and impaired Dhatu Agni.
Obesity and metabolic syndrome
In postmenopausal women, adipose tissue becomes the primary site for estrogen production through aromatization. This results in continuous low-grade estrogen exposure and chronic inflammatory signaling, both of which increase the likelihood of atypical hyperplasia. Insulin resistance further activates IGF-1 pathways that stimulate epithelial proliferation and stromal fibrosis, making metabolic correction central to recurrence prevention.
Chronic liver dysfunction
The liver is responsible for estrogen metabolism and detoxification. Non-alcoholic fatty liver disease, drug-induced hepatic stress, and impaired metabolic clearance lead to circulating estrogen excess and persistent fibrocystic changes. In Ayurvedic interpretation this corresponds to Yakrit Dushti and Ranjaka Pitta imbalance affecting Rakta Dhatu quality.
Hyperprolactinemia
Elevated prolactin levels are associated with mastalgia, duct ectasia, cyst formation, and nipple discharge. Even mild prolactin elevation can create a proliferative glandular environment and long-standing breast congestion.
Less Common but Clinically Significant Associations
Autoimmune disorders
Autoimmune thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, and Sjögren syndrome are associated with higher breast density and chronic inflammatory mastalgia. Persistent immune activation alters stromal signaling and epithelial turnover, increasing the likelihood of proliferative lesions.
Diabetes mellitus and insulin resistance
Chronic hyperinsulinemia promotes cellular proliferation through insulin-like growth factor pathways and is associated with increased breast density and higher atypia risk. Advanced glycation end products further contribute to stromal fibrosis and microvascular compromise.
Chronic stress and HPA-axis dysregulation
Long-standing cortisol imbalance alters gonadotropin release, ovarian function, prolactin response, and inflammatory cytokine activity. This produces cyclical mastalgia, breast congestion, and nodularity without a dominant structural lesion and is a major factor in recurrent benign breast symptoms.
Dense breast tissue
Extremely dense breast tissue is an independent risk factor for proliferative disease and interval cancers. It also increases the frequency of benign biopsies due to reduced imaging sensitivity and represents a hormonally active stromal environment.
Iodine deficiency
Low iodine status increases breast tissue sensitivity to estrogen and is associated with fibrocystic changes, nodularity, and persistent breast discomfort. This becomes clinically relevant in patients with coexisting thyroid dysfunction.
Rare but High-Significance Associations
Genetic cancer predisposition syndromes
In patients with strong family history, atypical hyperplasia may be a phenotypic marker of underlying mutations such as BRCA1, BRCA2, PALB2, CHEK2, or ATM. In such cases the lesion represents genomic instability rather than purely hormonal proliferation and requires high-risk surveillance protocols.
Chronic lymphatic congestion
Impaired lymphatic drainage due to prior surgery, radiation exposure, or prolonged sedentary lifestyle produces persistent nodularity, localized inflammatory changes, and breast heaviness, reflecting structural fluid stasis rather than true tumor formation.
Clinical Significance
Recognizing these systemic associations transforms management from local lump observation to comprehensive risk correction. When endocrine, metabolic, hepatic, immune, and lymphatic factors are addressed alongside structured imaging surveillance, the biological drivers of proliferative breast disease can be significantly reduced and long-term tissue stability improved.
What Should You Do After a Benign or Atypical Breast Diagnosis?
A diagnosis of a benign or atypical breast lesion is not the end of the evaluation. It is the beginning of a structured prevention and surveillance phase. The next step depends on the biopsy result, individual cancer risk, age, breast density, family history, and hormonal status. The goal is to prevent progression while avoiding unnecessary surgery.
If the lesion is non-proliferative
Non-proliferative findings such as simple cysts, mild fibrocystic change, or apocrine metaplasia usually require no surgical treatment. These patients enter a routine screening program with annual mammography after the age recommended for their country and periodic clinical breast examination. Symptom-directed follow-up is sufficient if the imaging and pathology are concordant.
If usual ductal hyperplasia or papilloma without atypia is reported
These lesions carry a mild increase in long-term cancer risk. Imaging follow-up is typically advised at regular intervals, and maintaining breast awareness becomes important. In patients with dense breasts, supplemental ultrasound may be recommended. The focus at this stage is risk reduction through metabolic, hormonal, and lifestyle correction.
If atypical ductal or lobular hyperplasia is diagnosed
Atypical hyperplasia places the patient in a high-risk surveillance category. Annual mammography is required, and in women with a calculated lifetime risk above the accepted threshold, breast MRI may be added. A detailed risk assessment using validated models helps determine whether preventive endocrine therapy should be discussed. Surgical excision may be advised if the core biopsy and imaging findings are not fully concordant.
If the biopsy result and imaging do not match
When radiology and pathology are discordant, excisional biopsy is recommended to ensure that a more advanced lesion has not been missed. This step is critical for patient safety and should not be delayed.
If there is a strong family history of breast or ovarian cancer
Patients with multiple affected relatives, early onset cancers in the family, or bilateral disease should undergo formal genetic risk evaluation. Identification of hereditary cancer syndromes changes both screening frequency and preventive strategy and may include MRI-based surveillance at an earlier age.
If the breasts are extremely dense
Dense breast tissue reduces mammographic sensitivity and independently increases cancer risk. These patients benefit from personalized screening protocols that may include tomosynthesis, ultrasound, or MRI depending on overall risk profile.
If the patient is premenopausal with cyclical breast symptoms
Cyclical pain and nodularity often reflect hormonal fluctuation rather than structural progression. These patients require symptom management, endocrine balance, and periodic imaging rather than repeated invasive procedures.
If the patient is postmenopausal
Any new palpable mass in a postmenopausal woman requires prompt imaging evaluation even if prior reports were benign, because background hormonal protection has declined.
Long-term prevention strategy
Once the immediate diagnosis is clarified, the patient moves into a long-term prevention plan. This includes maintaining optimal body composition, improving insulin sensitivity, regulating hormonal metabolism, supporting hepatic estrogen clearance, ensuring adequate micronutrient status, and following a structured imaging schedule. This phase is where progression risk can be significantly reduced.
Clinical Decision Pathway After a Benign or Atypical Breast Diagnosis
| Role of Integrative Risk Reduc | Future Cancer Risk | Future Cancer Risk | Role of Integrative Risk Reduction |
|---|---|---|---|
| Non-proliferative lesion (simple cyst, mild fibrocystic change) | No significant increase | Routine age-appropriate mammography and clinical breast exam | Hormonal balance, metabolic optimization, liver support for estrogen clearance |
| Usual ductal hyperplasia (UDH) | Mild increase (1.5–2×) | Annual imaging; breast awareness; ultrasound if dense breast | Weight regulation, insulin sensitivity, anti-inflammatory diet, lymphatic circulation support |
| Papilloma without atypia | Slight increase | Imaging surveillance; excision only if symptomatic or discordant | Prolactin regulation, ductal congestion reduction, endocrine stabilization |
| Atypical ductal hyperplasia (ADH) | Moderate to high (4–5×) | Annual mammography ± MRI; risk assessment models; consider preventive endocrine therapy | Tissue microenvironment correction, Kapha–Pitta modulation, immune surveillance support |
| Atypical lobular hyperplasia (ALH) | Moderate to high (4–5×, bilateral risk) | High-risk screening protocol; long-term surveillance | Systemic metabolic correction and Rasayana-based tissue resilience |
| Radiology–pathology discordance | Uncertain until excision | Surgical excision recommended | Post-diagnostic recovery and recurrence prevention strategies |
| Strong family history / genetic predisposition | High lifetime risk | Genetic counseling; MRI-based screening; earlier surveillance | Long-term inflammation control and Dhatu-level nourishment |
| Extremely dense breast tissue | Independent risk factor | Tomosynthesis, ultrasound, or MRI based on risk score | Hormonal metabolism correction and Meda Dhatu regulation |
| Premenopausal cyclical nodularity with benign imaging | Low structural risk | Symptom-based follow-up; avoid repeated invasive procedures | Cycle regulation, Apana Vata balance, stress axis stabilization |
| New palpable lump in postmenopausal patient | Higher baseline risk | Immediate diagnostic imaging and biopsy if indicated | Supportive care after structural diagnosis |
Ayurvedic Perspective & Cure
From an integrative oncology perspective, benign and atypical breast lesions represent a reversible biological phase in which chronic inflammation, Kapha-mediated tissue overgrowth, Pitta-driven cellular atypia, and Rakta and Meda Dhatu dushti alter the microenvironment of the breast. The therapeutic goal is not local lump reduction alone but systemic correction of hormonal metabolism, lymphatic drainage, hepatic detoxification pathways, and immune recognition of abnormal cells. This aligns with modern preventive oncology, which focuses on microenvironmental modulation rather than late-stage cytotoxic intervention.
Ayurveda interprets benign and atypical breast disorders as the result of disturbed dosha equilibrium, impaired dhatu metabolism, and blocked srotas. The breast (stana) is considered to be supported primarily by rasa dhatu, rakta dhatu, and meda dhatu. When Kapha accumulates, it causes glandular overgrowth; when Pitta is vitiated, inflammatory changes dominate; and when Vata obstructs circulation, irregular painful masses manifest. Such swellings are described in the classical literature under the categories of stana granthi (glandular swellings), arbuda moolavastha (premalignant tumorous states), and apachi (localized lymphatic swellings) as mentioned in Charaka Samhita (Chikitsa Sthana 7/30), Sushruta Samhita (Nidana Sthana 11), and Bhavaprakasha Nighantu [56][57].
Principles of Management
Ayurveda describes three therapeutic pillars for such conditions: shodhana (purification), shamana (pacification), and rasayana (rejuvenation). Shodhana eliminates the accumulated doshas and prevents further progression. Shamana corrects dosha vitiation through herbal and mineral formulations. Rasayana strengthens dhatus and prevents recurrence.
Classical Formulations for Benign and Atypical Breast Lesions
- Herbal formulations
- Kanchnar Guggulu (Bhaishajya Ratnavali, Granthi–Arbuda Adhikara) – reduces glandular swellings and is especially indicated for granthi and arbuda.
- Triphala Guggulu – detoxifying and rakta-shodhaka (blood purifier).
- Varunadi Kashaya – effective in cystic and fibrotic changes.
- Punarnavadi Kashaya – clears lymphatic congestion and kapha accumulation.
- Pushyanuga Churna (Ashtanga Hridaya) – useful in yoni vyapad and benign reproductive-tract swellings, often employed in breast nodularity.
- Kanchnar Guggulu (Bhaishajya Ratnavali, Granthi–Arbuda Adhikara) – reduces glandular swellings and is especially indicated for granthi and arbuda.
- Potent herbs
- Haridra (Curcuma longa) – anti-inflammatory, anti-proliferative.
- Guduchi (Tinospora cordifolia) – immunomodulatory and rasayana.
- Ashwagandha (Withania somnifera) – anti-stress, adaptogenic, hormone-modulating.
- Shigru (Moringa oleifera) – reduces fibrotic swelling.
- Nimba (Azadirachta indica) – detoxifying and kapha–pitta pacifying.
- Manjistha (Rubia cordifolia) – powerful rakta-shodhana herb.
- Haridra (Curcuma longa) – anti-inflammatory, anti-proliferative.
- Mineral and metallic preparations (Bhasma and Rasayana)
- Swarna Bhasma (gold calx) – rasayana, rejuvenates dhatus and strengthens immunity.
- Rajata Bhasma (silver calx) – cooling, kapha-pitta pacifying, useful in inflammatory breast swellings.
- Tamra Bhasma (copper calx) – potent in granthi and fibrotic changes.
- Trivanga Bhasma – balances kapha–vata disorders involving meda dhatu.
- Abhrak Bhasma (mica ash, mentioned in Rasendra Chintamani) – enhances tissue regeneration and strengthens rasa and rakta dhatus.
- Yashad Bhasma (zinc calx) – supports wound healing and epithelial stability.
- Godanti Bhasma (gypsum calx) – cooling, anti-inflammatory.
- Mukta Shukti Bhasma (pearl oyster shell) – pitta–kapha balancing, strengthens dhatus.
- Pravala Bhasma (coral calx) – pacifies pitta, indicated in nodular swellings.
- Lauh Bhasma (iron calx) – corrects rakta dushti and anemia-related tissue weakness.
- Sphatika Bhasma (alum calx) – astringent, reduces secretions and swelling.
- Heerak Bhasma (diamond ash) – mentioned in Rasaratna Samuccaya as a supreme rasayana, strengthens immunity and prevents malignant transformation.
- Ras Sindoor and Tal Sindoor – mercurial preparations indicated in resistant granthi conditions (only under specialist supervision).
- Sila Sindoor and Mall Sindoor – described in Rasatarangini as effective in stubborn kapha–pitta granthi.
- Swarna Bhasma (gold calx) – rasayana, rejuvenates dhatus and strengthens immunity.
- Compound Rasayana formulations
- Vyadhiharan Rasayana – supports overall immunity, strengthens tissue response.
- Poorna Chandrodaya Ras – potent rasayana mentioned in Rasendra Chintamani for difficult granthi–arbuda.
- Suvarna Parpati – rejuvenative and stabilizing rasayana preparation.
- Ras Raj Ras – indicated in arbuda and pre-cancerous conditions.
- Vyadhiharan Rasayana – supports overall immunity, strengthens tissue response.
Panchakarma Therapies
Virechana (purgation) is classically recommended for clearing pitta and rakta toxins. Basti (medicated enema) addresses vata obstruction and ensures smooth circulation of rasa and rakta in stana. Raktamokshana (bloodletting) is advised in localized pitta–rakta dushti, as per Sushruta Samhita (Sutra Sthana 14). These therapies are carefully selected according to the doshic predominance and patient strength.
Diet and Lifestyle
Ayurveda emphasizes pathya (wholesome regimen) and apathya (to be avoided). Patients are advised to consume warm, light, and easily digestible foods while avoiding excess dairy, fried items, red meat, and sweets, which aggravate Kapha and Meda. Fresh vegetables, bitter greens, turmeric, garlic, and cruciferous foods are emphasized for their kapha-shamaka and rakta-shodhana effects. Regular abhyanga with medicated oils like Kumkumadi Taila or Kshirbala Taila is recommended to keep breast channels open and prevent srotas avarana. Stress reduction through yoga, meditation, and pranayama such as Anuloma Viloma and Bhramari is also integral.
Modern Integrative View
Scientific studies increasingly validate Ayurvedic wisdom. Curcumin has shown inhibitory effects on epithelial hyperplasia and proliferative activity [58]. Ashwagandha extracts have demonstrated apoptotic effects on abnormal breast epithelial cells [59]. Guduchi is now studied for its immunomodulatory and anti-tumor properties [60]. Minerals like nano-formulations of Swarna and Rajata bhasma have shown potent antioxidant and immune-regulating effects in experimental models [61][62]. These findings underscore the relevance of integrating classical therapies with modern preventive oncology.
Management Principles
The management of benign and atypical breast lesions requires a tailored strategy that balances reassurance, surveillance, and intervention. While many benign conditions are self-limiting and carry minimal long-term risk, proliferative lesions with or without atypia require closer monitoring and, in some cases, definitive intervention. Modern oncology emphasizes risk-based stratification and precision diagnostics, while Ayurveda focuses on restoring systemic equilibrium through dosha balancing, detoxification, and rasayana therapies. Together, these approaches offer a comprehensive care model that addresses both pathology and constitutional susceptibility.
Modern medical approach
Non-proliferative lesions such as simple cysts and fibrocystic changes are usually managed conservatively. Symptom relief may involve analgesics, dietary adjustments, and reassurance, with surgical drainage considered only for recurrent or painful cysts. Proliferative lesions without atypia are managed with excision only if symptomatic or if imaging suggests architectural distortion. Otherwise, routine follow-up and lifestyle modification are advised.
Atypical hyperplasias, particularly atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH), are often excised due to the risk of concurrent carcinoma. Women with atypia are enrolled in high-risk screening programs involving annual mammography and, in selected cases, breast MRI. Chemoprevention with selective estrogen receptor modulators such as tamoxifen or raloxifene is considered for women with elevated lifetime risk. Genetic counseling is recommended where family history suggests hereditary cancer syndromes.
Ayurvedic management
Ayurveda approaches management by addressing the root cause of dosha imbalance and correcting tissue-level pathology. In non-proliferative lesions, therapies focus on Kapha pacification and maintaining srotas patency. Mild proliferative lesions are treated with a combination of shodhana (such as virechana or basti) and shamana formulations including Kanchnar Guggulu, Triphala Guggulu, and Varunadi Kashaya. Atypical proliferative lesions require more intensive interventions, combining panchakarma purification, dietary regulation, and the use of rasayana formulations such as Swarna Bhasma, Abhrak Bhasma, and Guduchi Rasayana under strict medical supervision.
Classical references recommend the use of specific therapies for breast swellings. Sushruta Samhita describes raktamokshana (bloodletting) for localized inflammatory or proliferative swellings, while Bhaishajya Ratnavali lists Kanchnar Guggulu as a cornerstone therapy for granthi and arbuda. Rasashastra texts such as Rasatarangini recommend metallic preparations like Tamra Bhasma and Rajata Bhasma for fibrotic or nodular swellings, emphasizing their capacity to reduce kapha accumulation and stabilize dhatu metabolism.
Diet and lifestyle regulation
Both modern and Ayurvedic management stress the importance of lifestyle modification. Patients are advised to maintain a healthy weight, minimize alcohol consumption, avoid prolonged use of hormone replacement therapy, and practice regular physical activity. In Ayurveda, dietary regulation involves Kapha-pacifying foods, reduced intake of dairy and fried foods, and incorporation of bitter, pungent, and astringent tastes to counter tissue overgrowth. Yoga, pranayama, and meditation are recommended to regulate hormonal cycles and reduce the psychoneuroendocrine influence on breast tissue health.
Integrative care model
An integrative framework allows patients to benefit from the precision of modern oncology while drawing upon the preventive and systemic strength of Ayurveda. For example, a patient with atypical hyperplasia may undergo surgical excision and high-risk screening while simultaneously adopting Ayurvedic detoxification, rasayana therapy, and lifestyle regimens to reduce recurrence and improve quality of life. This dual approach emphasizes long-term prevention, immune resilience, and holistic well-being, creating a care model that is both evidence-based and constitutionally individualized.
These interventions are not positioned as a replacement for imaging surveillance or biopsy when clinically indicated. Instead, they function as a long-term risk-reduction strategy aimed at improving tissue resilience, metabolic stability, and immune surveillance while the patient remains under structured medical follow-up.
Lesser-Known Facts for Patients
Most patients are familiar with the idea that breast lumps may be harmless or, in some cases, precancerous. However, there are many subtler aspects of benign and atypia breast conditions that remain poorly understood outside clinical and academic circles. Awareness of these lesser-known facts can reduce fear, improve compliance with follow-up, and empower women to take preventive steps.
Not all lumps are the same
Many women assume that all palpable lumps must be malignant. In reality, the majority of breast lumps in women under 40 are benign. Simple cysts can even fluctuate with the menstrual cycle, enlarging premenstrually and shrinking afterward. Such dynamic changes are not usually a sign of cancer, but they can cause unnecessary worry if not explained clearly.
Risk can be bilateral, even if the lesion is unilateral
In atypical hyperplasia, risk is not confined to the affected breast. Both breasts carry elevated lifetime risk, which is why long-term monitoring must cover both sides. This fact is often overlooked and leads some women to under-prioritize surveillance of the contralateral breast.
Imaging may not always give certainty
Even advanced imaging such as mammography or MRI cannot always distinguish benign proliferative lesions from early cancer. A biopsy is often needed for confirmation. Patients should be reassured that biopsy is not a sign that cancer is suspected, but rather an essential diagnostic step to ensure clarity.
Family history is not the only factor
While family history is important, many women with atypia and no family history still develop cancer, while others with strong family history never do. Risk is a combination of histology, lifestyle, hormonal exposures, and genetic background. Patients should not feel falsely reassured or unduly alarmed based solely on family history.
Lifestyle makes a measurable difference
Modern studies show that obesity, alcohol use, and prolonged hormone therapy increase the risk of progression from atypia to malignancy. Conversely, regular exercise, maintaining a healthy weight, and a diet rich in fruits, vegetables, and anti-inflammatory foods reduce risk. From an Ayurvedic perspective, these same preventive measures correspond to reducing Kapha aggravation, purifying Rakta, and maintaining balanced agni (digestive fire).
Ayurveda explains tissue behavior in unique ways
In Ayurvedic pathology, benign swellings correspond to early dosha disturbances, particularly Kapha-induced overgrowth or Pitta-mediated inflammation. Atypia is understood as deeper dhatu vitiation, where Rakta and Meda dhatus become unstable. This conceptualization allows interventions like Panchakarma cleansing and Rasayana rejuvenation to be seen not only as supportive but also preventive, aiming to stabilize tissues before malignant transformation occurs.
Emotional health matters
Stress and unresolved psychological strain can alter hormonal balance, immune response, and even breast tissue behavior. Modern psycho-oncology emphasizes this mind–body link, while Ayurveda has long advocated meditation, yoga, and pranayama as essential for preventing dosha imbalance. Patients should be encouraged to view stress management as an integral part of breast health, not a luxury.
Some benign conditions require lifelong awareness
Conditions such as atypical ductal hyperplasia do not “go away” once excised. The underlying tissue biology confers a permanent elevated risk. Long-term surveillance, rather than short-term reassurance, is key. Ayurveda addresses this reality through ongoing Rasayana therapy, dietary regulation, and seasonal detoxification, aligning with the concept of chronic preventive care.
Patients diagnosed with benign or atypical breast lesions often ask what they should do next. The most important steps include entering a structured surveillance program, understanding individual risk category, correcting metabolic and hormonal drivers, maintaining optimal body composition, and following a long-term breast health plan rather than seeking one-time treatment.
Frequently Asked Questions (FAQs)
What does a benign breast lump mean?
A benign breast lump is a non-cancerous growth. Most are caused by cysts, fibroadenomas, or fibrocystic change. They do not spread to other parts of the body, but some types may slightly increase future breast cancer risk, which is why proper imaging follow-up is important.
Is a benign breast lump a sign of cancer?
No. Most benign lumps are not cancer. However, certain biopsy findings such as atypical hyperplasia indicate a higher lifetime risk. Your radiology report and pathology result together determine whether routine screening or high-risk surveillance is needed.
Does atypical hyperplasia turn into cancer?
Atypical hyperplasia is not cancer, but it increases the risk of developing breast cancer in the future by about four to five times. With structured screening, risk assessment, and preventive care, many women never develop cancer.
What happens after a benign breast biopsy?
After a benign biopsy, your doctor checks whether the imaging and pathology match. If they are concordant, you usually return to routine screening. If atypia or discordance is present, you may need closer imaging follow-up, risk evaluation, or surgical excision.
Do all benign breast lumps need to be removed?
No. Most benign breast lumps do not require surgery. Removal is only advised if the lump grows, causes symptoms, shows atypical cells, or if imaging and biopsy findings do not match.
How often should screening be done after atypical hyperplasia?
Women with atypical hyperplasia usually need annual mammography. If their lifetime breast cancer risk is high, breast MRI may also be recommended. The exact schedule depends on age, breast density, and family history.
What is the cancer risk with dense breasts?
Dense breast tissue slightly increases breast cancer risk and makes mammograms harder to interpret. Many women with dense breasts benefit from supplemental screening such as ultrasound or MRI based on their overall risk profile.
Can breast cancer risk be reduced naturally?
Yes. Maintaining a healthy weight, improving insulin sensitivity, regulating hormonal balance, limiting alcohol, and following an anti-inflammatory lifestyle can significantly reduce long-term breast cancer risk when combined with regular screening.
Is breast pain a sign of breast cancer?
Breast pain alone is rarely a sign of cancer. It is usually related to hormonal fluctuation, cysts, or fibrocystic change. However, persistent focal pain with a new lump should always be evaluated with imaging.
When should I see a breast specialist?
You should see a specialist if you feel a new lump that persists beyond one menstrual cycle, notice nipple discharge, develop skin dimpling, or receive a biopsy report showing atypia or discordant findings.
References
- Charaka, Agnivesha. (2018). Charaka Samhita (Chikitsa Sthana 7/30). Varanasi: Chaukhambha Sanskrit Sansthan (Reprint).
- Sushruta, Dalhana. (2015). Sushruta Samhita (Nidana Sthana 11). Varanasi: Chaukhambha Orientalia.
- Vagbhata. (2012). Ashtanga Hridaya (Nidana Sthana 15). Varanasi: Chaukhambha Krishnadas Academy.
- Bhavamishra. (2016). Bhavaprakasha Nighantu (Koshthanga Rogadhikara). Varanasi: Chaukhambha Bharati Academy.
- Sharma, S. (2017). Bhaishajya Ratnavali (Granthi–Arbuda Adhikara). Varanasi: Chaukhambha Sanskrit Series.
- Sharma, Sadananda. (2016). Rasatarangini. Delhi: Motilal Banarsidass.
- Nityananda. (2015). Rasendra Chintamani. Varanasi: Chaukhambha Orientalia.
- Namboodiri, K. (2014). Rasaratna Samuccaya. Kerala: Kerala Ayurveda Academy.
- Hartmann, L. C., Sellers, T. A., Frost, M. H., et al. (2005). Benign breast disease and the risk of breast cancer. New England Journal of Medicine, 353(3), 229–237. https://doi.org/10.1056/NEJMoa044383
- Collins, L. C., Baer, H. J., Tamimi, R. M., et al. (2006). Family history and breast cancer risk in women with benign breast disease. Cancer, 107(6), 1240–1247. https://doi.org/10.1002/cncr.22106
- Dupont, W. D., & Page, D. L. (1985). Risk factors for breast cancer in women with proliferative breast disease. New England Journal of Medicine, 312(3), 146–151. https://doi.org/10.1056/NEJM198501173120303
- Degnim, A. C., Visscher, D. W., Berman, H. K., et al. (2007). Stratification of breast cancer risk in women with atypia: Mayo cohort study. Journal of Clinical Oncology, 25(19), 2671–2677. https://doi.org/10.1200/JCO.2006.08.3070
- Hartmann, L. C., Radisky, D. C., Frost, M. H., et al. (2014). Premalignant potential of atypical hyperplasia: Longitudinal cohort data. Breast Cancer Research, 16(5), 502. https://doi.org/10.1186/s13058-014-0502-8
- Schnitt, S. J. (2003). Benign breast disease and breast cancer risk: Morphologic and molecular considerations. American Journal of Surgical Pathology, 27(6), 836–847. https://doi.org/10.1097/00000478-200306000-00013
- Page, D. L., Schuyler, P. A., Dupont, W. D., et al. (1985). Atypical hyperplastic lesions of the breast: Long-term follow-up. Cancer, 55(11), 2698–2708. https://doi.org/10.1002/1097-0142(19850601)55:11<2698::AID-CNCR2820551132>3.0.CO;2-2
- Collins, L. C., Carlo, V. P., Hwang, H., & Schnitt, S. J. (2006). Intraductal papillomas: Associations with atypia and carcinoma in situ. Human Pathology, 37(7), 802–808. https://doi.org/10.1016/j.humpath.2006.02.018
- Colditz, G. A., Rosner, B. A., & Speizer, F. E. (1996). Risk factors and family history in breast cancer. JNCI, 88(6), 365–371. https://doi.org/10.1093/jnci/88.6.365
- Worsham, M. J., Raju, U., Lu, M., et al. (2009). Risk factors from benign breast disease in diverse populations. Breast Cancer Research and Treatment, 118(1), 1–7. https://doi.org/10.1007/s10549-009-0301-9
- Masood, S. (2008). Proliferative lesions in the breast: Diagnostic and prognostic spectrum. Pathology Research and Practice, 204(7), 483–496. https://doi.org/10.1016/j.prp.2008.03.001
- American College of Radiology (ACR). (2013). BI-RADS® Atlas (5th ed.). https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems/BI-RADS
- U.S. Preventive Services Task Force. (2024). Final recommendation statement: Screening for breast cancer. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
- National Comprehensive Cancer Network (NCCN). (2025). Breast cancer screening and diagnosis (Version 2.2025). https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1421
- American Cancer Society. (2023). Breast cancer screening guidelines. https://www.cancer.org/cancer/types/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html
- Monticciolo, D. L., Malak, S. F., Friedewald, S. M., et al. (2023). Screening women at higher risk: Updated recommendations. Journal of the American College of Radiology, 20(10), 1031–1045. https://doi.org/10.1016/j.jacr.2023.05.001
- American College of Radiology. (2023). Practice parameter for breast MRI. https://gravitas.acr.org/PPTS/GetDocumentView?docId=123
- American College of Radiology. (2019–2020). Practice parameter for breast ultrasound examination. https://gravitas.acr.org/PPTS/DownloadPreviewDocument?DocId=125
- Marinovich, M. L., Hunter, K. E., Macaskill, P., et al. (2018). Tomosynthesis vs mammography: Meta-analysis. JNCI, 110(9), 942–949. https://doi.org/10.1093/jnci/djy099
- Conant, E. F., Barlow, W. E., Herschorn, S. D., et al. (2019). Digital breast tomosynthesis vs mammography. JAMA Oncology, 5(5), 635–642. https://doi.org/10.1001/jamaoncol.2018.7078
- Sharpe, R. E., Venkataraman, S., Phillips, J., et al. (2016). Increased detection with DBT. Radiology, 278(3), 698–707. https://doi.org/10.1148/radiol.2015150370
- Li, T., Marinovich, M. L., & Houssami, N. (2022). Density-specific benefits of DBT vs DM. Breast, 63, 52–61. https://doi.org/10.1016/j.breast.2022.01.001
- Warner, E., Messersmith, H., Causer, P., et al. (2008). MRI screening of high-risk women: Meta-analysis. Annals of Internal Medicine, 148(9), 671–679. https://doi.org/10.7326/0003-4819-148-9-200805060-00009
- Berg, W. A., Zhang, Z., Lehrer, D., et al. (2012). MRI and ultrasound in high-risk women (ACRIN 6666). JAMA, 307(13), 1394–1404. https://doi.org/10.1001/jama.2012.388
- Ding, W., Wang, Y., Liu, D., et al. (2023). MRI vs mammography in high-risk women: Meta-analysis. Medicine, 102(10), e32903. https://doi.org/10.1097/MD.0000000000032903
- Chung, W.-S., Pak, S. H., Park, S.-W., et al. (2024). Contrast-enhanced mammography: Review. Diagnostics, 14(14), 1553. https://doi.org/10.3390/diagnostics14141553
- Nissan, N., Zittan, E., et al. (2024). Diagnostic accuracy of contrast-enhanced mammography. Radiology. https://doi.org/10.1148/radiol.232580
- Tian, H., Sun, H., Guo, Q., et al. (2024). Core needle biopsy vs excision. World Journal of Surgical Oncology, 22(1), 198. https://doi.org/10.1186/s12957-024-03452-7
- Okoli, C., Anyanwu, S., et al. (2020). Ultrasound-guided core biopsy in resource-limited settings. Nigerian Journal of Clinical Practice, 23(6), 771–778. https://doi.org/10.4103/njcp.njcp_102_20
- Park, V. Y., Kim, E.-K., Kim, M. J., et al. (2017). Imaging-pathology concordance in biopsy. Ultrasonography, 36(4), 341–349. https://doi.org/10.14366/usg.17021
- Park, H. L., & Kim, L. S. (2014). Vacuum-assisted biopsy: Indications and outcomes. Int J Med Sci, 11(8), 811–818. https://doi.org/10.7150/ijms.8777
- Zhu, Y., Yu, J., et al. (2023). Vacuum-assisted biopsy systems: Review. Frontiers in Oncology, 13, 1205696. https://doi.org/10.3389/fonc.2023.1205696
- Tu, S., Wang, J., Li, S., et al. (2023). Intraductal papilloma without atypia: Management. Gland Surgery, 12(4), 402–411. https://doi.org/10.21037/gs-22-426
- Wahab, R. A., Xu, M., et al. (2021). Upgrade rate of pure FEA. Radiology: Imaging Cancer, 3(4), e210116. https://doi.org/10.1148/rycan.2021200116
- Ferré, R., Radecka, B., et al. (2022). Upgrade of percutaneous FEA: Review. Breast, 62, 134–141. https://doi.org/10.1016/j.breast.2022.01.014
- Polat, D. S., Kılıç, F., et al. (2023). Radial scars without atypia: Surveillance vs excision. Eur J Breast Health, 19(1), 1–7. https://doi.org/10.4274/ejbh.galenos.2022.2022-6-3
- Trombadori, C. M. L., Amabile, M. I., et al. (2021). Radial scar: Management dilemma. Annals of Breast Surgery, 5, 26. https://doi.org/10.21037/abs-20-117
- Kuba, M. G., Salih, A. M., et al. (2023). Update on lobular lesions. Pathobiology, 90(5), 265–281. https://doi.org/10.1159/000529877
- Dabbs, D. J., Chivukula, M., et al. (2015). E-cadherin IHC in breast pathology. Human Pathology, 46(10), 1438–1446. https://doi.org/10.1016/j.humpath.2015.05.005
- Rakha, E. A., & Ellis, I. O. (2011). E-cadherin as diagnostic biomarker. Diagnostic Pathology, 6, 36. https://doi.org/10.1186/1746-1596-6-36
- Christgen, M., & Derksen, P. W. B. (2020). Invasive lobular carcinoma: Differences vs ductal. Breast Cancer Research, 22, 51. https://doi.org/10.1186/s13058-020-01384-6
- Visscher, D. W., Pankratz, V. S., & Hartmann, L. C. (2010). Ki-67 as biomarker in atypia. Breast Cancer Res Treat, 121(2), 431–437. https://doi.org/10.1007/s10549-009-0584-x
- Gail, M. H., Brinton, L. A., Byar, D. P., et al. (1989). Individualized breast cancer risk projection. JNCI, 81(24), 1879–1886. https://doi.org/10.1093/jnci/81.24.1879
- Tyrer, J., Cuzick, J., & Sasieni, P. (2004). Breast cancer prediction model. Statistics in Medicine, 23(7), 1111–1130. https://doi.org/10.1002/sim.1668
- Lee, A., Mavaddat, N., Wilcox, A. N., et al. (2019). BOADICEA risk model. Genetics in Medicine, 21(8), 1708–1718. https://doi.org/10.1038/s41436-018-0406-9
- U.S. Preventive Services Task Force. (2019). Breast cancer: Medications for risk reduction. JAMA, 322(9), 857–867. https://doi.org/10.1001/jama.2019.11885
- Visvanathan, K., Fabian, C. J., Bantug, E., et al. (2019). Endocrine therapy for risk reduction: ASCO guideline. JCO, 37(33), 3152–3165. https://doi.org/10.1200/JCO.19.01472
- Tiwari, S., Dwivedi, S., & Sharma, A. (2011). Concept of granthi and arbuda in Ayurveda: Correlation with breast disease. AYU, 32(2), 242–246. https://doi.org/10.4103/0974-8520.92578
- Choudhary, A., Singh, R., & Sharma, V. (2015). Ayurvedic perspective of stana roga (breast disorders): A review. Journal of Ayurveda and Integrative Medicine, 6(2), 75–82. https://doi.org/10.4103/0975-9476.146547
- Aggarwal, B. B., & Harikumar, K. B. (2009). Curcumin’s therapeutic potential against breast cancer. Breast Cancer, 16(4), 273–280. https://doi.org/10.1007/s12282-009-0112-9
- Widodo, N., Takagi, Y., Shrestha, B. G., et al. (2010). Selective killing of cancer cells by Withania somnifera leaf extract. PLoS ONE, 5(10), e13536. https://doi.org/10.1371/journal.pone.0013536
- Singh, S., & Patel, D. K. (2020). Tinospora cordifolia (Guduchi): A reservoir for therapeutic applications. Indian Journal of Traditional Knowledge, 19(4), 812–824. http://nopr.niscair.res.in/handle/123456789/55611
- Jain, A., Chauhan, P., Meena, H., & Sharma, R. (2015). Abhrak Bhasma and its nanoformulations: Pharmacological profile. Ancient Science of Life, 34(3), 153–159. https://doi.org/10.4103/0257-7941.157149
- Singh, R., & Pandey, B. L. (2021). Swarna Bhasma in integrative cancer care: Evidence and directions. Journal of Ethnopharmacology, 277, 114265. https://doi.org/10.1016/j.jep.2021.114265