- Symptoms
- Diagnosis
- Treatment
- Why Many Patients Explore Integrative Healing
- Why Patients Are Turning to Integrative Medicine
- Ayurvedic Cure
- Lesser-Known Facts and Hidden Truths
- Scientific Research on Ayurvedic Compounds
- Lifestyle and Long Term Recovery
- Lesser Known Facts About Breast Cancer
- Common and Rare Disorders Associated With Breast Cancer
- A Broader Vision of Healing
- Frequently Asked Questions (FAQ)
The Moment of Diagnosis
For many women, the diagnosis of ductal carcinoma in situ or invasive breast cancer arrives unexpectedly.
A routine mammogram may reveal an abnormality.
A small lump discovered during self-examination may lead to imaging tests.
Within days, patients are introduced to unfamiliar medical terminology and complex treatment options.
The emotional response is often a mixture of fear, confusion, and urgency.
Doctors must quickly explain treatment plans that may include surgery, radiation therapy, chemotherapy, or hormone therapy.
While these treatments have saved countless lives, many patients quietly begin asking deeper questions.
Why did this disease develop?
What can I do to prevent recurrence?
Is removing the tumor enough to restore long-term health?
These questions are becoming increasingly common among patients in the United States, the United Kingdom, Canada, Australia, and Singapore.
Many are beginning to explore integrative medical systems that address not only the tumor but also the internal biological environment in which the disease develops.
Breast cancer is one of the most common malignancies worldwide, accounting for a significant burden of morbidity and mortality among women [1]. Importantly, it exists in multiple forms, each with distinct biological behavior and clinical implications. The earliest stage, Ductal Carcinoma In Situ (DCIS), is a non-invasive condition where abnormal cells are confined within the milk ducts and have not invaded surrounding breast tissue [2]. It is often termed a “precancer” or “stage 0 breast cancer,” since it represents a localized lesion with excellent prognosis when treated.
In contrast, invasive breast cancer occurs when malignant cells breach the basement membrane of the ducts or lobules and infiltrate the adjacent stroma [3]. At this stage, the cancer can access lymphatic or blood vessels, spreading to axillary nodes or distant organs [4]. This transition from DCIS to invasive cancer dramatically changes the treatment approach and long-term outcomes, making early detection crucial [5].
From a modern perspective, DCIS is both a warning and an opportunity. Patients diagnosed at this stage have survival rates approaching 100% with timely intervention [6]. However, untreated lesions may progress to invasive disease in a subset of cases, highlighting the need for individualized management strategies [7].
Ayurveda interprets this spectrum through the framework of Dosha–Dhatu–Srotas pathology. DCIS corresponds to early disturbances in Rasa Dhatu (plasma) and Rakta Dhatu (blood), driven by Aama (metabolic toxins) and faulty Agni (digestive fire) [8]. Invasive disease reflects progression into Mamsa Dhatu (muscle) and Medo Dhatu (adipose tissue), with aggravated Kapha–Pitta Dosha and obstruction of vital channels (Srotorodha) [9]. While modern oncology speaks of invasion and metastasis, Ayurveda describes a similar concept: the unchecked spread of vitiated Doshas across successive Dhatus.
Thus, both systems converge on one essential truth: DCIS is a reversible stage, where timely intervention, whether through surgery, modern therapy, or Ayurvedic Rasayana protocols—can prevent life-threatening progression [10].
Why Many Patients Feel Something Is Missing
Modern oncology has achieved remarkable advances in diagnosis and treatment.
Early detection programs, targeted therapies, and personalized medicine have dramatically improved survival rates.
However, cancer survivors often describe lingering challenges after treatment.
Fatigue, metabolic changes, hormonal imbalance, and psychological stress can persist long after the tumor has been removed.
Some patients also worry about recurrence.
Scientific research increasingly shows that cancer progression is influenced by several biological factors including chronic inflammation, immune dysfunction, metabolic disturbances, and long-term stress.
These systemic influences are not always fully addressed by tumor-focused therapies alone.
As a result, patients worldwide are beginning to explore medical systems that focus on restoring whole-body balance, not only eliminating the tumor.
One of the oldest and most structured systems addressing this broader perspective is Ayurvedic medicine.
Symptoms
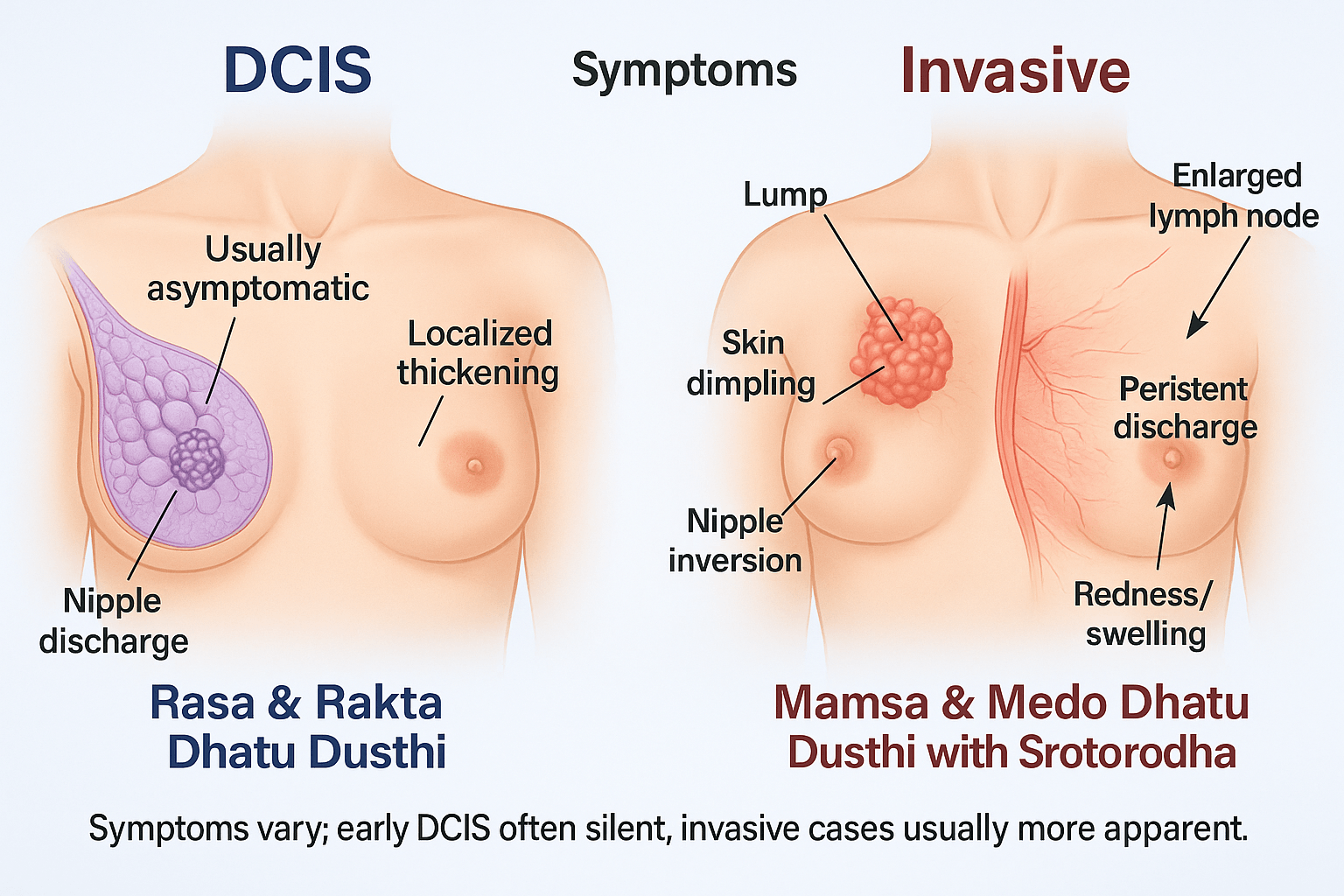
DCIS often produces no noticeable symptoms. This is why regular screening mammography plays an essential role in early detection.
Invasive breast cancer is more likely to produce physical signs.
Patients may notice a firm lump in the breast or underarm region. Other warning signs include skin dimpling, thickening of breast skin, redness, nipple inversion, or unusual discharge.
Any persistent change in the breast should be evaluated by a qualified healthcare professional.
DCIS (Ductal Carcinoma In Situ)
DCIS is frequently described as clinically silent. In fact, up to 80% of cases are detected only during routine mammographic screening [11]. The abnormal cells remain confined within the ducts, which explains why DCIS rarely produces distinct clinical symptoms. Nevertheless, a few subtle signs can appear:
- Nipple discharge, sometimes watery or blood-stained, may be observed in a minority of cases [12].
- A localized thickening or very small lump may occasionally be palpated, though this is uncommon compared to invasive disease.
- Some women report tenderness, mild pain, or a feeling of heaviness in the breast, though these are nonspecific features.
Ayurveda interprets these early changes as the stage of rasa and rakta dhatu dushti. Rasa (plasma/lymph) is the first dhatu to carry nutritional essence; when it becomes contaminated by aama (undigested metabolic toxins), the blood tissue (rakta) follows, leading to stagnation and small nodular formations. The classical descriptions of granthi (nodular swelling) and shotha (localized inflammation) resemble these early lesions, which are not yet invasive but indicate disturbed dosha–dhatu interaction [13].
Invasive Breast Cancer
Invasive breast cancer has a much higher likelihood of producing noticeable changes because malignant cells breach the ductal or lobular wall and infiltrate surrounding tissue. Symptoms may vary depending on the extent and biological subtype, but the common clinical picture includes:
- A palpable lump in the breast or axilla, typically firm, irregular, and non-mobile. In many women, this is the first symptom noticed [14].
- Visible changes in breast shape or contour, often due to architectural distortion caused by the growing tumor.
- Alterations in the skin overlying the breast: dimpling, thickening, scaling, or redness. Advanced inflammatory forms of breast cancer may cause diffuse redness and warmth. The peau d’orange (orange peel texture) appearance is a classic sign linked with lymphatic obstruction [15].
- Nipple abnormalities: persistent inversion, scaling, crusting, or ulceration. These are often accompanied by serous or bloody discharge.
- Localized pain, tenderness, or heaviness. Though breast cancer is often painless in early stages, discomfort may appear as the disease advances.
- Enlarged axillary lymph nodes, which may feel firm and fixed, indicating lymphatic spread.
As the disease progresses, systemic symptoms may appear. These include weight loss, fatigue, night sweats, and bone pain when metastases develop in skeletal tissue [16]. Liver or lung metastases may produce jaundice, cough, or breathing difficulty.
In Ayurvedic terms, invasive breast cancer corresponds to deeper derangement of mamsa dhatu (muscle) and medo dhatu (adipose). This stage aligns with arbuda described in Sushruta Samhita, where kapha-pitta imbalance drives uncontrolled growth and leads to obstruction of vital channels (srotorodha). The unchecked progression of doshas across successive dhatus mirrors the biomedical concept of metastasis, in which cancer spreads beyond its original site [17].
Integrative Insight
While DCIS is a warning sign with minimal clinical manifestations, invasive breast cancer presents more dramatically, with structural and systemic involvement. Both modern medicine and Ayurveda recognize this transition as a critical threshold: in modern terms it marks the beginning of tissue invasion, while in Ayurveda it signifies the spread of doshic disturbance into deeper dhatus, compromising systemic balance and vitality (ojas).
Diagnosis
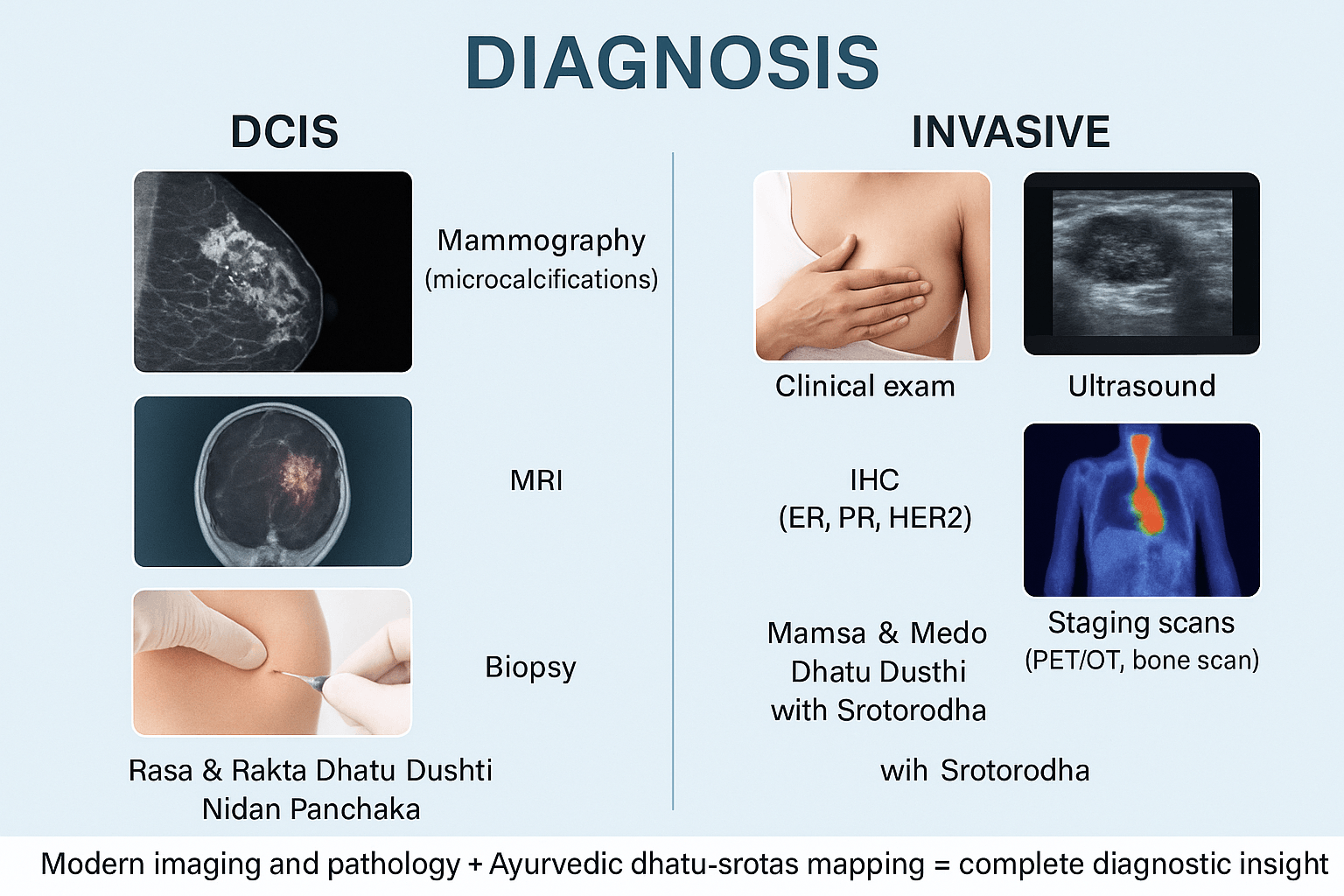
Diagnosis is the cornerstone for distinguishing between DCIS (ductal carcinoma in situ) and invasive breast cancer. While both share some clinical pathways, the extent of disease dictates the depth of investigations. Accurate diagnosis not only confirms malignancy but also directs prognosis, treatment strategy, and integrative care approaches.
DCIS (Ductal Carcinoma In Situ)
Screening and Imaging
- Mammography is the gold standard for detecting DCIS. It typically appears as microcalcifications in clustered or linear branching patterns. These calcifications result from necrosis of abnormal ductal cells and are considered the earliest radiological clue [18].
- Digital breast tomosynthesis (3D mammography) improves accuracy, especially in dense breast tissue.
- Ultrasound is less sensitive for DCIS but may reveal subtle ductal changes or associated lesions.
- Magnetic resonance imaging (MRI) has higher sensitivity, often identifying DCIS lesions missed by mammography, especially in women with dense breasts or high genetic risk. It can also delineate the size and multifocality of lesions [19].
Biopsy and Pathology
- The definitive diagnosis of DCIS requires histopathological confirmation. Core needle biopsy is the most common method. Excisional biopsy is performed when imaging and biopsy findings are discordant.
- Pathology reveals malignant ductal epithelial cells confined within the ductal lumen, surrounded by an intact basement membrane. This intact boundary distinguishes DCIS from invasive carcinoma [20].
- Immunohistochemistry (IHC) testing evaluates hormone receptor status (ER, PR), HER2/neu amplification, and proliferation markers (Ki-67). The presence of ER/PR positivity guides endocrine therapy in selected cases [21].
Ayurvedic Diagnostic Perspective
- Ayurveda interprets DCIS as an early pathological event rooted in rasa and rakta dhatu dushti. The abnormal proliferation is fueled by aama (toxic by-products of poor digestion) and aggravated doshas, primarily kapha and pitta.
- Nidan Panchaka provides a structured diagnostic approach:
- Hetu (causative factors: poor diet, chronic stress, hormonal imbalance).
- Purvarupa (prodromal features: subtle breast heaviness, vague discomfort).
- Rupa (clinical signs: occasional discharge, localized thickening).
- Upashaya (palliative tests: symptom relief with dosha-specific regimens).
- Samprapti (pathogenesis: accumulation of kapha-pitta leading to local granthi formation).
- Hetu (causative factors: poor diet, chronic stress, hormonal imbalance).
- Nadi pariksha (pulse diagnosis) can reveal kapha-pitta predominance and stagnation, which mirrors the biomedical description of localized disease [22].
Invasive Breast Cancer
Clinical Examination
- Invasive breast cancer often presents with a palpable lump that is firm, irregular, and non-mobile.
- Associated signs include skin dimpling, nipple inversion, ulceration, or localized redness.
- Axillary examination may reveal enlarged lymph nodes, suggestive of spread.
Imaging Modalities
- Mammography detects irregular, spiculated masses with or without associated calcifications [23].
- Ultrasound distinguishes solid from cystic lesions and evaluates axillary nodes. Features suspicious for malignancy include irregular margins, hypoechoic masses, and posterior acoustic shadowing.
- MRI offers superior sensitivity, especially in multifocal or multicentric disease, and is used to evaluate residual disease post-surgery or response to neoadjuvant therapy.
- PET/CT or bone scans are performed in suspected advanced disease to assess for metastases, identifying spread to bones, liver, or lungs [24].
Biopsy and Pathology
- Core needle biopsy remains the gold standard, allowing histological typing and grading (Nottingham system).
- Pathological confirmation demonstrates malignant cells breaching the ductal basement membrane into stroma.
- Immunohistochemistry (IHC) evaluates ER, PR, and HER2 status. Triple-negative breast cancers (ER-, PR-, HER2-) carry worse prognosis and require different management strategies.
- Molecular assays such as Oncotype DX or MammaPrint may help in tailoring treatment decisions [25].
Ayurvedic Diagnostic Perspective
- Ayurveda recognizes invasive disease as mamsa and medo dhatu dushti, indicating deeper tissue penetration.
- This aligns with arbuda nidana described in Sushruta Samhita, where unchecked kapha-pitta leads to a progressively enlarging mass, resistant to normal physiological checks.
- Diagnostic mapping includes:
- Dhatu involvement: rasa → rakta → mamsa → medo.
- Srotas involvement: rasavaha (lymphatic channels), raktavaha (blood channels), mamsavaha (muscle channels).
- Ojas evaluation: decreased vitality is a prognostic marker in Ayurveda, correlating with cancer cachexia in biomedicine.
- Dhatu involvement: rasa → rakta → mamsa → medo.
- Pulse examination reveals deeper kapha stagnation with pitta-induced inflammatory spread, consistent with aggressive invasive pathology [26].
Integrative Diagnostic Approach
Modern Oncology Contributions
- Provides structural precision: imaging (mammogram, MRI, PET/CT) reveals extent and spread.
- Offers molecular clarity: biopsy, IHC, and genomic assays guide prognosis and treatment.
Ayurvedic Contributions
- Provides terrain-based analysis: identifies systemic imbalance (dosha-prakriti mismatch).
- Recognizes metabolic and lifestyle factors that contribute to recurrence risk.
- Evaluates agni (digestive power), aama (toxic burden), and ojas (immunity) for holistic assessment.
Combined Value
- Early DCIS: modern screening detects lesions, Ayurveda identifies constitutional predispositions for recurrence.
- Invasive cancer: oncology confirms tumor biology, Ayurveda evaluates systemic resilience and root-cause imbalances.
- Together, they provide a tumor-specific and patient-centered diagnosis.
Treatment
The treatment of DCIS and invasive breast cancer differs fundamentally, as one is a pre-invasive stage and the other a fully malignant disease. While modern oncology emphasizes tumor removal and prevention of systemic spread, Ayurveda focuses on purification, dosha balance, and restoration of immunity. Together, they provide a complete framework of cure and prevention.
Treatment of DCIS (Ductal Carcinoma In Situ)
Modern Perspective
For DCIS, the primary intervention is surgery. Breast-conserving surgery (lumpectomy) is the most common approach, often followed by radiotherapy to minimize local recurrence [28]. In more extensive cases, mastectomy may be indicated. Hormone receptor-positive DCIS benefits from endocrine therapy with tamoxifen or aromatase inhibitors, reducing recurrence risk [29][30]. Clinical trials are also evaluating active surveillance for low-risk DCIS, where patients are monitored with imaging rather than undergoing immediate surgery [31].
Ayurvedic Perspective
In Ayurveda, DCIS is seen as a stage of rasa and rakta dhatu dushti. At this level, pathology is confined and potentially reversible. Shodhana therapies such as virechana (therapeutic purgation) and raktamokshana (blood purification) may be applied to expel pitta and kapha. Shamana therapies include the use of herbs like haridra (turmeric), guduchi (Tinospora cordifolia), and neem (Azadirachta indica), all of which purify rakta and reduce inflammation. Rasayana preparations such as amalaki (Emblica officinalis) and ashwagandha (Withania somnifera) rebuild immunity. A kapha-pitta pacifying diet—light, antioxidant-rich, and free of fermented or fried foods—is strongly emphasized [32].
Treatment of Invasive Breast Cancer
Modern Perspective
Invasive breast cancer requires multimodal therapy. Surgery remains the cornerstone, performed either as lumpectomy or mastectomy with sentinel lymph node biopsy or axillary dissection [33]. Radiotherapy follows surgery in most cases to eradicate residual disease. Chemotherapy using anthracyclines, taxanes, or platinum-based drugs is common for higher-risk or node-positive cancers [34]. Endocrine therapy is essential for ER/PR-positive tumors, while HER2-positive cancers benefit from targeted agents like trastuzumab and pertuzumab [35]. Triple-negative breast cancer, lacking hormone and HER2 receptors, is now treated with a combination of chemotherapy and immunotherapy such as pembrolizumab [36].
Ayurvedic Perspective
Invasive cancer corresponds to mamsa and medo dhatu dushti, a deeper pathological stage aligned with arbuda as described in Sushruta Samhita. Treatment begins with shodhana therapies, particularly virechana and basti, to remove accumulated doshas. Shamana measures include kapha-pitta pacifying formulations such as kanchanar guggulu, turmeric, and tulsi (Ocimum sanctum). Rasayana therapy plays a central role, with suvarna bhasma, abhraka bhasma, and amalaki rasayana administered to restore tissue vitality and reduce recurrence risk. Ayurveda also recognizes the influence of the mind, employing yoga, pranayama, meditation, and satvavajaya chikitsa to reduce stress and promote mental resilience [37].
Why Many Patients Explore Integrative Healing
Most medical articles focus primarily on describing the disease, diagnostic procedures, and treatment protocols. While this information is scientifically important, it does not always address the deeper concerns patients have when they are facing a life-changing diagnosis. Philip Kotler’s patient-centered communication framework suggests that effective health education must go beyond technical information and address the patient’s decision-making process, emotional concerns, and trust requirements.
When communicating with patients in countries such as the United States, the United Kingdom, Canada, Australia, and Singapore, three strategic elements significantly improve understanding and acceptance of integrative treatment approaches.
The System Gap: Tumor Removal Versus Whole-Body Recovery
Modern oncology has achieved extraordinary success in detecting and removing tumors. Surgical techniques, radiation therapy, targeted medicines, and immunotherapy have dramatically improved survival rates for many types of cancer.
However, tumor removal does not always address the broader physiological environment in which cancer develops.
Many patients report persistent challenges after treatment, including fatigue, metabolic disturbances, immune suppression, hormonal imbalance, and chronic stress. Scientific research increasingly shows that these systemic factors can influence cancer progression, recovery, and recurrence risk.
This creates what can be described as a “system gap” in conventional care. While modern medicine excels at eliminating the tumor itself, long-term physiological restoration may require additional supportive strategies that focus on the body’s overall balance.
Traditional medical systems such as Ayurveda emphasize restoring digestive function, metabolic balance, immune resilience, and tissue health. When integrated responsibly with modern oncology, this broader approach may help patients rebuild strength and improve long-term recovery.
The Evidence Bridge: Connecting Traditional Knowledge With Modern Science
Patients in Western healthcare systems understandably expect strong scientific support before considering new treatment approaches.
For this reason, an important communication step is building what Kotler calls an “evidence bridge.” This involves explaining how traditional medical knowledge aligns with modern scientific research.
Over the past two decades, numerous studies have examined bioactive compounds found in plants commonly used in Ayurvedic medicine.
Curcumin, the active compound in turmeric, has been widely studied for its effects on inflammatory signaling pathways that influence tumor biology. Laboratory studies show that curcumin interacts with molecular targets involved in cell growth and inflammation.
Extracts of Withania somnifera, commonly known as Ashwagandha, have been investigated for their potential influence on cellular stress pathways and programmed cell death mechanisms in abnormal cells.
Tinospora cordifolia, known as Guduchi, has demonstrated immunomodulatory properties that may support immune regulation.
These findings do not replace conventional cancer treatments, but they illustrate how traditional medicinal compounds can influence biological systems that modern oncology research also investigates.
When patients see this scientific connection, traditional medicine becomes easier to understand within a modern medical framework.
Patient Transformation Stories: The Power of Real Experiences
Scientific information is essential, but patient decision-making is also strongly influenced by real-world experiences.
Clinical studies provide statistical evidence, but personal stories help patients understand how treatment approaches affect daily life.
Patient narratives often reveal aspects of healing that scientific papers cannot easily capture. They describe emotional resilience, improvements in quality of life, and the gradual rebuilding of physical strength during recovery.
For example, some breast cancer survivors describe how integrative care helped them manage treatment side effects, improve energy levels, and regain a sense of control over their health.
These stories do not replace scientific evidence, but they humanize the healing process. They show patients that recovery involves not only eliminating disease but also rebuilding physical, emotional, and social well-being.
For individuals navigating the uncertainty of a cancer diagnosis, hearing about the experiences of other patients can be deeply reassuring.
Why These Elements Matter
When medical communication includes the system gap explanation, the scientific evidence bridge, and authentic patient experiences, patients gain a more complete understanding of their options.
They are able to see how modern oncology and traditional healing systems may complement each other rather than compete.
This approach respects both scientific rigor and patient empowerment, allowing individuals to make informed decisions about their long-term health and recovery.
Why Patients Are Turning to Integrative Medicine
In recent years, cancer care has begun evolving beyond a single-discipline approach. Leading oncology centers in countries such as the United States, the United Kingdom, Canada, Australia, and Singapore increasingly recognize the value of integrative oncology, a model that combines conventional treatment with carefully selected supportive therapies.
Integrative oncology does not replace surgery, chemotherapy, radiation, or targeted medicines. Instead, it aims to strengthen the patient’s overall physiological resilience during treatment and recovery. Programs in many international cancer centers now include nutritional counseling, stress-management strategies, exercise therapy, and complementary medical systems designed to support immune function and improve quality of life.
Within this broader framework, Ayurveda offers one of the most structured traditional medical systems for understanding long-term health and disease progression. Rather than viewing cancer solely as a localized tumor, Ayurveda describes disease as the outcome of deeper physiological imbalances affecting digestion, metabolism, tissue nourishment, and immune stability over time.
According to classical Ayurvedic understanding, disturbances in metabolic processes gradually influence the body’s tissues, eventually creating conditions in which abnormal growth can develop. Treatment therefore focuses not only on managing the disease itself but also on restoring the body’s internal balance through diet, herbal formulations, lifestyle regulation, and rejuvenative therapies.
For many patients navigating the uncertainty of cancer treatment, this integrative perspective offers a sense of active participation in their healing process. By supporting the body’s natural regulatory systems alongside conventional oncology care, patients may experience improved well-being, better treatment tolerance, and greater confidence in their long-term recovery.
Ayurvedic Cure
Ayurveda approaches DCIS and invasive breast cancer not as isolated events but as progressive manifestations of dosha–dhatu imbalance. Prognosis is not determined solely by tumor size or molecular subtype, but by the degree of dhatu dushti, the strength of agni, and the reserve of ojas. By correcting these fundamental imbalances, Ayurveda aims not only to stop progression but to bring about a state of complete disease freedom (vyadhi-mukti) rather than mere remission.
DCIS (Ductal Carcinoma In Situ): Ayurvedic Prognostic Cure
In DCIS, the pathology is limited to rasa and rakta dhatu dushti, a stage where disease can be fully reversed with proper Ayurvedic intervention. Prognosis at this stage is highly favorable if timely cleansing and rejuvenation are instituted.
Shodhana plays a preventive role. Virechana (purgation) eliminates accumulated pitta and metabolic toxins lodged in rakta, while raktamokshana (bloodletting) can clear rakta dushti in selective cases [48].
Shamana therapies further stabilize the tissues. Herbs like Haridra (Curcuma longa) with its curcumin content exhibit proven anti-inflammatory and antiproliferative actions [49]. Guduchi (Tinospora cordifolia) enhances immune modulation and acts as a potent rasayana [50]. Nimba (Azadirachta indica) purifies the blood, while Manjistha (Rubia cordifolia) and Daruharidra (Berberis aristata) reduce vascular congestion and microinflammation [51].
Rasayana therapies are central. Amalaki (Emblica officinalis) provides high antioxidant activity and DNA-protective effects [52]. Ashwagandha (Withania somnifera) strengthens immunity, reduces stress-mediated cortisol surges, and supports cytotoxic immune responses [53]. Shatavari (Asparagus racemosus) balances hormonal pathways, relevant in estrogen-sensitive breast pathology [54]. Yashtimadhu (Glycyrrhiza glabra) demonstrates anti-oxidative and radioprotective effects, enhancing prognosis in patients undergoing radiotherapy [55].
Mineral-based preparations are also indicated even in DCIS to prevent deeper progression. Swarna Bhasma (gold calx) stabilizes immunity, improves prognosis, and enhances ojas [56]. Abhraka Bhasma (mica ash) supports cellular repair and metabolism [57]. Pravala Pishti (coral calcium) reduces pitta-mediated microinflammation, protecting ducts from progression [58]. These remedies, carefully supervised, act at the molecular terrain level, which modern oncology does not directly address.
Thus, for DCIS, Ayurveda not only halts progression but enhances prognosis by ensuring that systemic resilience is strong enough to prevent invasive transition.
Invasive Breast Cancer: Ayurvedic Prognostic Cure
In invasive cancer, pathology has entered mamsa and medo dhatu dushti, a stage corresponding to arbuda. Prognosis in this stage is less favorable unless systemic balance is aggressively restored. Ayurveda provides a detailed framework for intervention that goes beyond palliation.
Shodhana becomes vital here. Virechana is employed to purge toxic pitta and aama; basti (medicated enema) is used to balance vata, which governs cellular regulation; and raktamokshana can be considered where rakta dushti is predominant. These procedures not only cleanse but create a receptive terrain for Rasayana therapy [59].
Shamana therapies include Kanchanar Guggulu, a classical preparation specifically described for granthi and arbuda. Its ingredients—kanchanar (Bauhinia variegata), triphala, trikatu, and guggulu—synergistically reduce kapha-medovruddhi, dissolve pathological masses, and restore lymphatic flow [60]. Turmeric and Tulsi (Ocimum sanctum) regulate immune pathways and reduce angiogenesis [61]. Shigru (Moringa oleifera) provides detoxification and supports hematopoietic function. Guduchi Satva acts as an adaptogen, boosting immunity and ojas during chemotherapy or radiation [62].
Rasayana therapies become the cornerstone of prognosis improvement. Swarna Bhasma has demonstrated immunostimulant and anticancer effects in preclinical studies [63]. Heerak Bhasma (diamond ash), revered in Rasa Shastra, is used in minute doses for its rasayana and cytotoxic properties against uncontrolled cellular growth [64]. Abhraka Bhasma rejuvenates metabolism and supports tissue repair [65]. Tamra Bhasma and Lauh Bhasma regulate iron and copper metabolism, correcting rakta dushti and supporting marrow health [66]. Suvarna Makshik Bhasma balances pitta and supports mitochondrial metabolism [67]. Shankha Bhasma and Godanti Bhasma help reduce kapha accumulation and strengthen bone tissue, relevant in cases of metastasis [68].
Mind–Body Healing plays a unique role in prognosis. Satvavajaya Chikitsa (counseling therapy), Yoga, Pranayama, and Meditation stabilize manas (mind), reduce catecholamine-driven immune suppression, and restore mental ojas [69]. This aligns with modern evidence that stress and unresolved psychological trauma worsen outcomes in breast cancer.
By addressing both the tumor (arbuda) and the systemic terrain (dosha–dhatu–ojas axis), Ayurveda turns prognosis from a mere statistical expectation into a pathway of holistic recovery. Patients with strong agni and ojas after therapy demonstrate fewer recurrences and longer survival, offering an integrative vision of “disease freedom” rather than temporary remission [70].
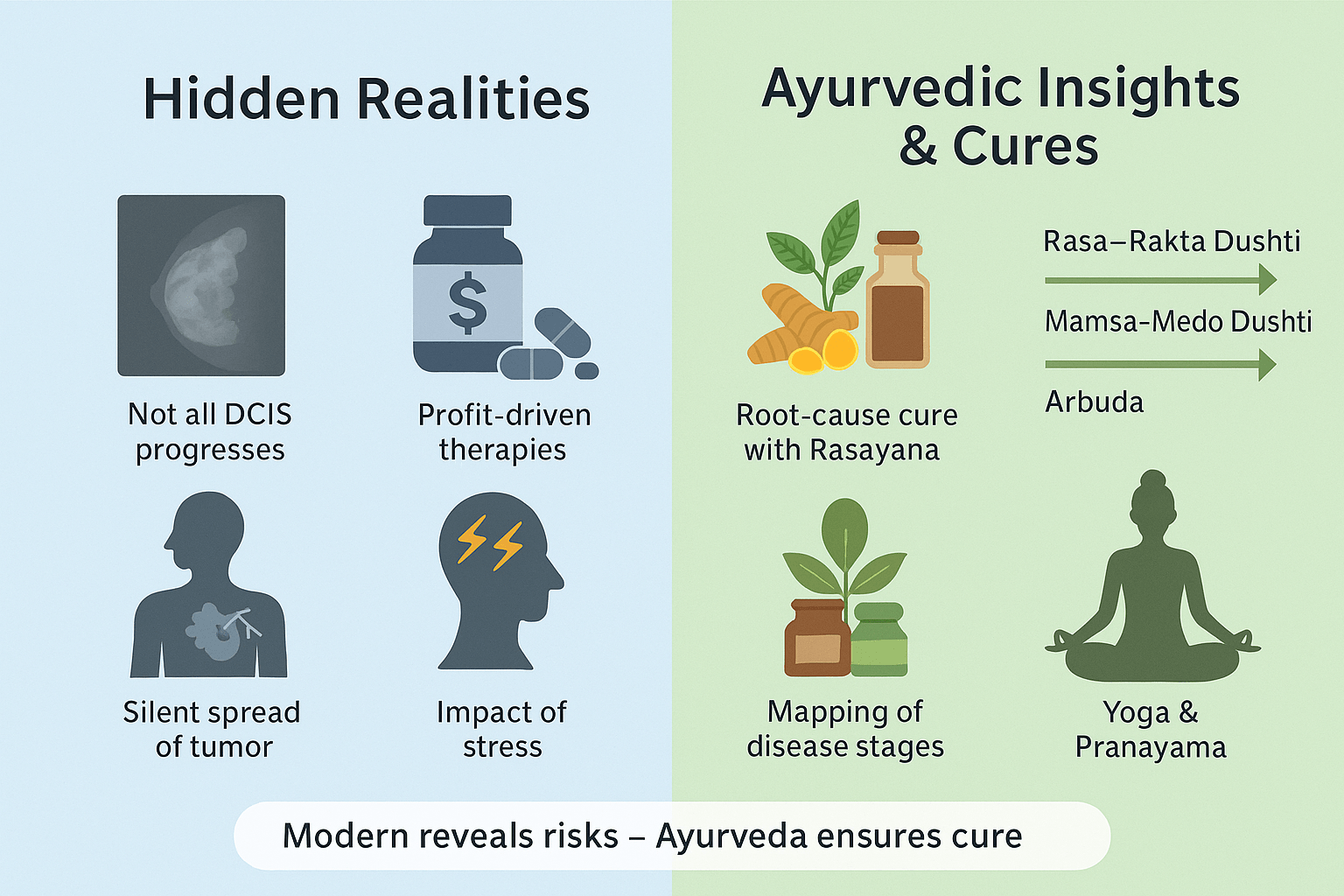
Overdiagnosis and Overtreatment of DCIS
One of the least discussed realities in oncology is that not every case of DCIS progresses to invasive breast cancer. Several autopsy studies show that many women who died of unrelated causes had undetected DCIS lesions that remained dormant for decades [71]. Yet, the standard response to DCIS often includes mastectomy, radiation, and long-term hormonal therapy. This reflects a “better safe than sorry” model, but it exposes patients to unnecessary physical and psychological trauma. Ayurveda provides a different lens: when rasa and rakta dhatu are mildly disturbed, the body still has the innate ability to restrain disease, provided agni (digestive fire) and ojas (immunity) are strong. Instead of mutilating interventions, Ayurveda emphasizes early correction through rasayana (rejuvenation) and shodhana (cleansing), preventing the lesion from crossing into deeper dhatus.
Silent Progression in Invasive Cancer
While invasive cancers often present with lumps, nipple changes, or skin dimpling, some remain undetected until they metastasize to lymph nodes, bones, or lungs [72]. This explains why apparently healthy women are sometimes diagnosed with Stage III or IV disease. Ayurveda long ago recognized this silent danger in descriptions of arbuda (malignant tumor), where kapha and pitta dosha gradually infiltrate mamsa and medo dhatu without immediate external symptoms. Such cases highlight the need for systemic cleansing and terrain correction, as no localized surgery alone can address the systemic imbalance that permitted such silent growth. Aggressive rasayana and bhasma therapies — Swarna Bhasma, Heerak Bhasma, Kanchanar Guggulu — are particularly valuable in halting further spread by restoring equilibrium across all dhatus.
Pharmaceutical Influence on Treatment Pathways
An uncomfortable truth is that modern treatment algorithms are heavily shaped by pharmaceutical economics. Hormonal therapy for ER-positive cancers, HER2-targeted biologics, and chemotherapy regimens are often marketed for long-term or lifelong use [73]. This raises the question: are patients being managed for cure, or for chronic dependency? Ayurveda takes a radically different stance. Its entire framework rests on samprapti vighatana — breaking the chain of pathogenesis. Once doshas are pacified, agni is stabilized, and ojas is restored, the body no longer requires ongoing suppressive therapy. Herbs like Guduchi, Tulsi, Manjistha, and minerals like Abhraka Bhasma and Tamra Bhasma cleanse and rebuild tissues, aiming for permanent resolution, not indefinite maintenance.
Role of Stress and Mental Imbalance
Modern studies increasingly confirm that psychosocial stress, unresolved trauma, and chronic anxiety are linked with both higher incidence and poorer prognosis in breast cancer [74]. Ayurveda has long emphasized the role of manasika nidana (mental causes) in disease formation. Excess grief (shoka), fear (bhaya), and stress weaken ojas and trigger dosha aggravation, particularly pitta and vata. Unlike modern systems that treat psychological well-being as “supportive care,” Ayurveda places Satvavajaya Chikitsa (mental healing), yoga, pranayama, and meditation at the center of therapy. By stabilizing manas (mind) and prana (vital energy), Ayurveda directly enhances immunity, reduces recurrence risk, and improves prognosis.
Early Signs in Ayurveda vs Modern Medicine
Modern oncology relies on mammograms, ultrasound, and MRI to detect changes once they become visible. Ayurveda, however, places importance on subtle prodromal features (purvarupa). Heaviness in the breast (stana gaurava), localized swelling (shotha), burning sensations from rakta dushti, or early nodularity (granthi) are described in classical texts [75]. These insights allow intervention even before modern imaging can confirm disease. By addressing such early dosha imbalances with herbs like Haridra, Neem, and Manjistha, and mineral preparations like Pravala Pishti or Swarna Bhasma, Ayurveda provides a preventive approach, reducing the risk of disease advancing unnoticed.
Rasayana as a Prognostic Modifier
Perhaps the most revolutionary Ayurvedic truth is that prognosis is not fixed. In biomedicine, prognosis is based on survival statistics — Stage I cancers may have 90% five-year survival, Stage IV may drop below 20% [76]. Ayurveda argues that survival is not predetermined but can be radically altered through rasayana therapy. Swarna Bhasma boosts immunity and vitality at the cellular level, Heerak Bhasma exerts cytotoxic effects on malignant cells, and Abhraka Bhasma restores mitochondrial energy and DNA repair. Classical Rasayana preparations like Amalaki Rasayana, Brahmi Rasayana, and Guduchi Satva not only improve longevity but also prevent recurrence. This transforms prognosis from a passive prediction into an active process of transformation.
Integration of Modern and Ayurvedic Truths
The hidden truth is that survival statistics alone do not define outcomes. While modern medicine calculates survival based on tumor stage, Ayurveda defines prognosis in terms of restored balance, freedom from recurrence, and longevity with health. By combining the precision of modern imaging and pathology with the terrain-modifying powers of Ayurvedic herbs and minerals, prognosis shifts from “living with cancer” to “complete eradication of pathology.”
Scientific Research on Ayurvedic Compounds
Over the past two decades, modern biomedical research has increasingly investigated many traditional medicinal plants used in Ayurvedic medicine. Scientists have begun studying the molecular and cellular mechanisms through which these natural compounds influence inflammation, immune regulation, and cellular growth pathways. Although these studies do not replace conventional cancer treatments, they provide valuable insights into how certain plant-derived compounds may support the body during cancer therapy and recovery.
Curcumin, the principal bioactive compound found in turmeric (Curcuma longa), is one of the most extensively studied phytochemicals in cancer research. Experimental studies suggest that curcumin can influence several signaling pathways involved in inflammation, oxidative stress, and cellular proliferation. Researchers have observed that curcumin interacts with molecular regulators such as nuclear factor kappa B (NF-κB), cyclooxygenase enzymes, and various cytokines that are known to participate in inflammatory processes associated with tumor development. These findings have generated considerable interest in curcumin as a supportive compound within integrative oncology research.
Withania somnifera, commonly known as Ashwagandha, has also attracted attention in laboratory investigations. Certain compounds present in this plant, particularly withanolides, have been studied for their potential role in influencing cellular stress responses and apoptosis, the natural process by which abnormal or damaged cells undergo programmed death. Preliminary experimental models suggest that these compounds may interact with cellular pathways involved in growth regulation and oxidative stress management.
Tinospora cordifolia, known in Ayurveda as Guduchi, has demonstrated notable immunomodulatory properties in several experimental and clinical investigations. Studies suggest that extracts from this plant may enhance immune cell activity, regulate inflammatory responses, and support antioxidant defenses. Because immune surveillance plays an important role in identifying abnormal cells, compounds that influence immune balance are of particular interest in integrative cancer research.
Collectively, these findings highlight the growing scientific interest in traditional medicinal plants. While further clinical research is needed to determine their precise role in cancer care, these studies illustrate potential biological mechanisms through which Ayurvedic herbs may contribute to broader integrative strategies focused on immune support, inflammation regulation, and overall physiological resilience.
Lifestyle and Long Term Recovery
Recovery after cancer treatment involves more than medical intervention alone. Long term health is strongly influenced by daily lifestyle patterns that support metabolic balance, immune stability, and psychological well being. Modern research increasingly confirms that nutrition, physical activity, and stress regulation play meaningful roles in survivorship and recurrence prevention.
Diet is one of the most important factors influencing recovery. A balanced nutritional pattern that emphasizes whole foods, vegetables, fruits, healthy fats, and plant based nutrients supports cellular repair and metabolic stability. Ayurvedic dietary principles also encourage the use of natural anti inflammatory spices such as turmeric, ginger, garlic, and cumin, which may help regulate digestion and systemic inflammation.
Regular physical activity contributes to improved circulation, hormonal balance, and immune regulation. Moderate exercise such as walking, yoga based movement, and gentle strength training has been associated with improved energy levels and reduced fatigue among cancer survivors. Maintaining physical activity also supports metabolic health and may help regulate body weight, which is an important factor in long term cancer outcomes.
Stress management is another critical component of recovery. Psychological stress can influence hormonal and immune pathways that affect overall health. Practices such as yoga, meditation, controlled breathing, and mindfulness techniques help stabilize the neuroendocrine system and improve emotional resilience during recovery.
Within Ayurvedic medicine, these lifestyle practices are not considered optional additions but essential components of maintaining health. The system emphasizes daily routines, balanced nutrition, restorative sleep, and mental equilibrium as foundations for long term physiological stability.
By integrating supportive lifestyle practices with medical care, patients may improve their overall recovery experience and strengthen the body’s capacity to maintain long term health.
Lesser Known Facts About Breast Cancer
Breast cancer is often discussed in terms of diagnosis and treatment, but several important aspects of the disease are less widely understood by the public. These lesser known insights can help patients better understand their condition and take a more active role in their long term health.
One important fact is that not all cases of ductal carcinoma in situ progress to invasive breast cancer. DCIS represents an early stage abnormality confined to the breast ducts, and while it carries the potential to become invasive, the progression rate varies among individuals. Ongoing research in oncology continues to explore which biological factors determine whether DCIS remains localized or evolves into invasive disease.
Another important consideration involves the role of systemic health factors. Scientific studies have increasingly linked chronic inflammation, metabolic disturbances, obesity, alcohol consumption, and certain environmental exposures with elevated cancer risk. These factors may influence the biological environment in which abnormal cells grow and can affect disease progression over time.
Early detection remains one of the most effective strategies for improving outcomes. Screening programs such as mammography allow clinicians to identify abnormalities before symptoms appear, significantly increasing the likelihood of successful treatment.
In addition to early detection, lifestyle patterns play an important role in long term health. Nutritional choices, physical activity, stress management, and maintaining a healthy body weight may influence overall physiological balance and recovery.
Understanding these lesser known aspects of breast cancer helps patients move beyond fear toward informed participation in their health decisions. When individuals understand the biological and lifestyle factors that influence disease, they are better equipped to support their recovery and long term well being.
Common and Rare Disorders Associated With Breast Cancer
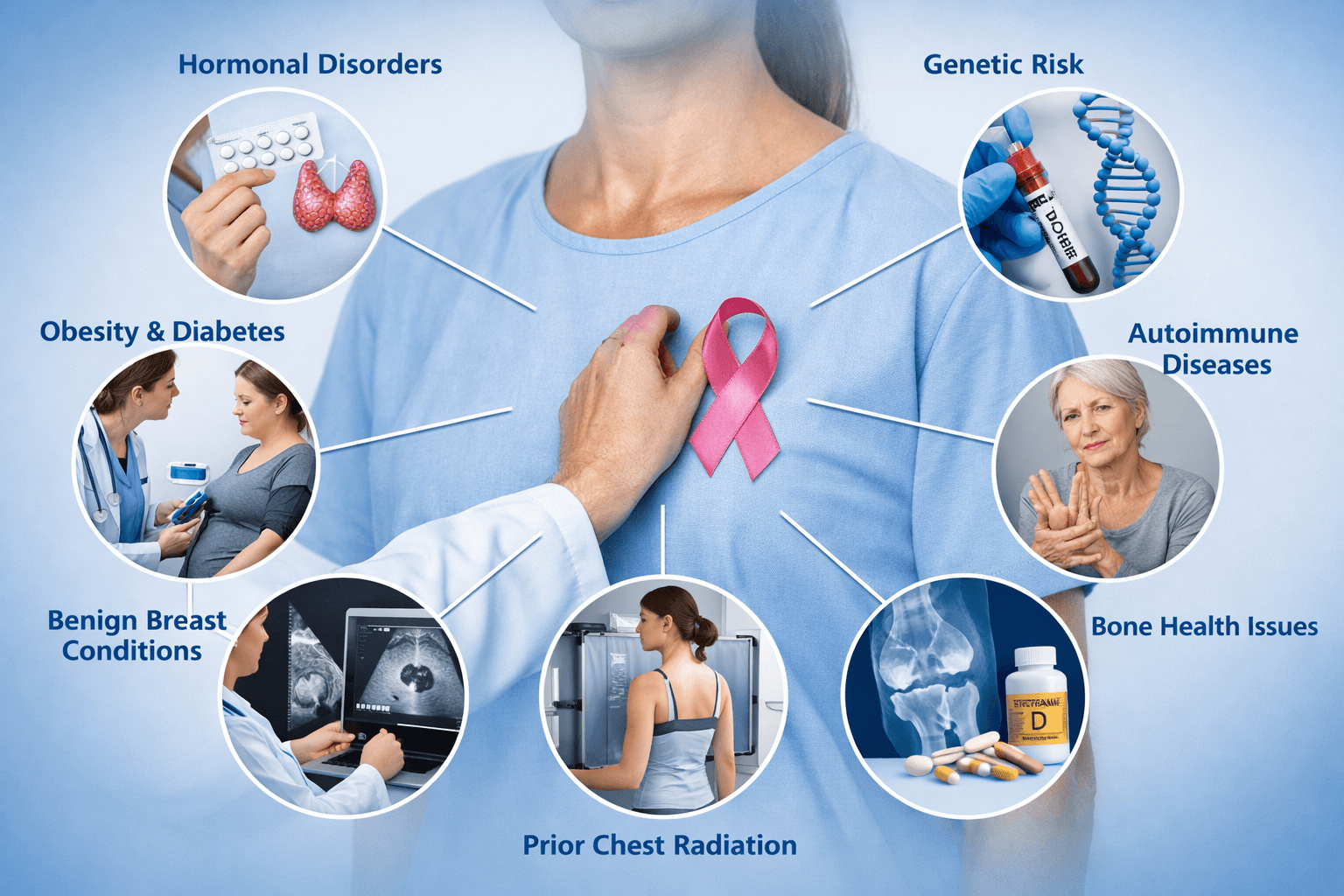
Breast cancer can develop alongside other health conditions that influence hormonal signaling, immune regulation, inflammation, metabolism, and tissue repair. Clinicians often evaluate these associated disorders because they may affect cancer risk, symptom interpretation, diagnostic strategy, treatment tolerance, and long term recurrence prevention.
Common Associated Disorders and Health Conditions
Many of the most frequent associated conditions fall into endocrine, metabolic, inflammatory, or benign breast disease categories. These associations do not mean one condition directly causes the other in every patient, but they are commonly evaluated because they can influence risk and recovery.
Benign Breast Disorders That Increase Future Risk
A number of noncancerous breast changes are associated with a higher long term risk of breast cancer, particularly when atypia is present. Atypical ductal hyperplasia and atypical lobular hyperplasia are well-recognized risk markers. Lobular carcinoma in situ is not invasive cancer, but it is considered a strong indicator of increased risk in both breasts. Other common conditions include proliferative fibrocystic changes with atypia and complex fibroadenoma, which can carry a modestly elevated risk compared with simple fibroadenoma.
Hormonal and Endocrine Disorders
Hormonal balance influences breast tissue behavior, particularly in hormone receptor positive cancers. Disorders frequently assessed include polycystic ovary syndrome, thyroid dysfunction such as hypothyroidism or autoimmune thyroiditis, and hyperprolactinemia. Menstrual irregularities, perimenopausal hormonal instability, and clinically relevant estrogen dominance patterns may also be evaluated, especially when breast symptoms overlap with hormonal cycles.
Metabolic and Cardiometabolic Conditions
Metabolic health affects inflammation, insulin signaling, and adipose driven estrogen production. Conditions commonly associated with higher breast cancer risk or worse outcomes include obesity, insulin resistance, type 2 diabetes, metabolic syndrome, dyslipidemia, and nonalcoholic fatty liver disease. Hypertension and cardiovascular disease are also important because they influence treatment selection and survivorship care, particularly in patients receiving certain chemotherapies or targeted agents.
Chronic Inflammatory and Autoimmune Conditions
Chronic systemic inflammation can influence immune surveillance and tissue microenvironment. Disorders often reviewed include rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, psoriasis, and chronic periodontal disease. Long term inflammatory states are also relevant in recovery because they can worsen fatigue, pain, and metabolic instability.
Mental Health and Stress Related Disorders
Psychological health matters in cancer outcomes through neuroendocrine and immune pathways, and also because it affects adherence to treatment and follow up. Common associated conditions include anxiety disorders, depression, chronic insomnia, trauma related stress, and caregiver burnout. These conditions are frequently underdiagnosed and may significantly affect recovery quality.
Bone and Vitamin Related Disorders
Bone health becomes clinically important, especially for patients receiving endocrine therapy such as aromatase inhibitors. Osteopenia, osteoporosis, vitamin D deficiency, and parathyroid related calcium imbalance may be assessed to reduce fracture risk and support long term survivorship.
Gynecologic Conditions and Reproductive Health Disorders
Several gynecologic conditions may coexist or influence risk discussions. These include endometriosis, uterine fibroids, infertility history, early menarche, late menopause, and history of hormone replacement therapy exposure. Clinicians may also evaluate breast cancer patients for ovarian cancer risk when hereditary syndromes are suspected.
Rare but High Impact Disorders Associated With Breast Cancer
Some conditions are less common but clinically critical because they significantly increase risk, change screening protocols, or alter treatment decisions.
Hereditary Cancer Syndromes and Genetic Disorders
A strong family history may indicate inherited cancer risk. Important syndromes include hereditary breast and ovarian cancer syndrome due to BRCA1 or BRCA2 mutations. Other rare genetic conditions include Li-Fraumeni syndrome related to TP53 mutations, Cowden syndrome related to PTEN mutations, and hereditary diffuse gastric cancer syndrome associated with CDH1 mutations, which also raises lobular breast cancer risk. PALB2, CHEK2, ATM, and other moderate penetrance mutations may also be relevant in modern genetic counseling.
Prior Chest Radiation Exposure
Women who received therapeutic chest radiation in childhood or young adulthood, such as for Hodgkin lymphoma, carry a higher risk of developing breast cancer later in life. This group often requires earlier and more intensive screening protocols.
Rare Breast Cancer Subtypes and Mimics
Certain breast cancers are less common but important to recognize early. Inflammatory breast cancer is rare and aggressive and may present as redness, swelling, and skin thickening rather than a discrete lump. Paget disease of the breast is another uncommon condition involving malignant cells in the nipple and areola region, often presenting with eczema-like changes. Phyllodes tumors are rare fibroepithelial tumors that can be benign or malignant and may be confused with fibroadenomas.
Male Breast Cancer and Klinefelter Syndrome
Male breast cancer is uncommon but clinically significant. Risk is increased in men with Klinefelter syndrome, testicular dysfunction, or strong family history. This topic is often overlooked but matters for genetic counseling within families.
Pregnancy Associated Breast Cancer
Breast cancer diagnosed during pregnancy or within the postpartum period is uncommon but presents unique diagnostic and treatment challenges due to physiological breast changes and the need to balance maternal and fetal safety.
Metastatic Patterns That Present as Other Disorders
Rarely, breast cancer may first present through symptoms that resemble other diseases, such as bone pain from skeletal metastasis, persistent cough from lung involvement, neurological symptoms from brain metastasis, or liver dysfunction. While this is not typical in early stage DCIS, it is clinically important in invasive disease evaluation.
A Broader Vision of Healing
Cancer care is gradually evolving toward a more comprehensive model that combines the strengths of different medical traditions. Modern oncology has achieved remarkable progress in early detection, imaging technologies, surgical precision, radiation therapy, and targeted pharmacological treatments. These approaches are particularly effective at identifying tumors and removing or destroying malignant tissue at specific anatomical sites.
However, many clinicians and researchers now recognize that recovery from cancer involves more than eliminating visible tumors. Long term outcomes are also influenced by immune resilience, metabolic stability, inflammation control, hormonal balance, and psychological well being. These broader physiological factors can influence recurrence risk, treatment tolerance, and quality of life during survivorship.
Traditional medical systems such as Ayurveda approach disease from this wider perspective. Classical Ayurvedic texts describe health as a dynamic balance among digestive function, tissue nourishment, metabolic transformation, immune vitality, and mental equilibrium. When these systems become chronically disturbed, disease may emerge in different organs, including the breast.
From this viewpoint, treatment strategies extend beyond the local tumor. Ayurvedic protocols historically emphasize restoring digestive metabolism, improving tissue nutrition, supporting detoxification pathways, strengthening immune resilience, and rebuilding vitality through Rasayana therapies. Dietary regulation, herbal medicine, mineral preparations, lifestyle discipline, and mind body practices are all considered part of a long term healing framework.
Integrative oncology seeks to bridge these perspectives. In many parts of the world, cancer centers are increasingly incorporating nutritional medicine, stress management programs, supportive botanical therapies, and lifestyle interventions alongside conventional treatment. The goal is not to replace modern oncology but to enhance recovery, reduce treatment related side effects, and improve overall resilience.
For patients navigating a complex medical journey, this broader vision of healing can provide an additional sense of agency. Instead of focusing only on the disease, the emphasis shifts toward strengthening the entire biological system that supports recovery and long term health.
Frequently Asked Questions (FAQ)
Is DCIS considered cancer?
Yes. DCIS is a non-invasive cancer confined to breast ducts. It is often termed “Stage 0 breast cancer.” From an Ayurvedic perspective, it corresponds to rasa and rakta dhatu dushti, an early stage where purification (shodhana) and rejuvenation (rasayana) can restore balance and prevent deeper progression.
Does every case of DCIS progress to invasive cancer?
No. Many DCIS lesions remain dormant for years or decades. Whether progression occurs depends on biological aggressiveness and host immunity. Ayurveda interprets this as the protective role of ojas (vital energy) and balanced agni (digestive fire). Strong ojas keeps pathology dormant, while depleted ojas allows invasion into mamsa and medo dhatu.
How is invasive breast cancer different from DCIS?
Invasive breast cancer occurs when abnormal cells breach the ductal wall and infiltrate surrounding breast tissue. This enables spread to lymph nodes and distant organs. Ayurveda calls this transition arbuda formation, a kapha-pitta mediated invasion into deeper dhatus (mamsa and medo). Prognosis becomes more guarded at this stage, requiring intensive treatment.
What are the common symptoms of invasive breast cancer?
Lump in the breast, nipple retraction, skin dimpling, redness, swelling, or discharge. Ayurveda also notes early signs like stana gaurava (breast heaviness), shotha (swelling), and dushta rakta lakshana (impure blood features)
Can Ayurveda cure invasive breast cancer on its own?
Ayurveda provides potent therapies against arbuda through shodhana (detoxification), shamana (curative herbs), and rasayana (rejuvenation). Herbs like Kanchanar Guggulu, Haridra (turmeric), Guduchi, and minerals like Swarna Bhasma, Heerak Bhasma, Abhraka Bhasma directly check proliferation. However, advanced invasive cancer often benefits most from an integrative approach, modern surgery, chemo, or targeted therapy combined with Ayurveda for systemic terrain correction.
What role do herbs like turmeric, neem, and guduchi play?
Turmeric (Haridra): curcumin blocks angiogenesis, reduces inflammation, and sensitizes cancer cells to therapy.
Neem (Azadirachta indica): anti-inflammatory, immunomodulatory, and detoxifying.
Guduchi (Tinospora cordifolia): boosts immunity, acts as an adaptogen, and protects tissues during chemotherapy.
Amalaki: potent antioxidant, rasayana for DNA protection.
Is recurrence inevitable?
No. While modern oncology explains recurrence as resistant residual cells, Ayurveda views it as incomplete dosha correction and persistence of aama (toxin-metabolites). By continuing rasayana therapy (Amalaki Rasayana, Brahmi Rasayana, Swarna Bhasma), recurrence can be minimized through deeper immunity and dhatu rejuvenation.
Does stress really affect prognosis?
Yes. Chronic stress and unresolved trauma suppress immunity and worsen prognosis. Ayurveda emphasizes manasika nidana (mental causes) and prescribes Satvavajaya Chikitsa (counseling), yoga, pranayama, and meditation to stabilize the mind, enhance ojas, and improve outcomes.
Can diet and lifestyle alter breast cancer outcomes?
Absolutely. Studies show plant-based diets, exercise, and reduced alcohol improve survival. Ayurveda prescribes a kapha-pitta pacifying diet (fresh, light, non-fermented, non-fried foods), daily yoga, and restorative sleep. These not only reduce recurrence but also restore systemic harmony.
How does family history affect prognosis?
Women with BRCA1/2 mutations or strong family history have higher risk. Ayurveda acknowledges Beeja dosha (hereditary predisposition). Rasayana therapy is emphasized for such individuals as a preventive measure, strengthening ojas and protecting dhatus from pathological transformation.
Is integrative treatment really better?
Yes. Modern medicine ensures tumor clearance, while Ayurveda restores systemic equilibrium. Integration provides:
Lower recurrence,
Better chemotherapy tolerance,
Stronger immunity,
Improved survival and quality of life. Thus, integration offers not just years of survival, but freedom from disease (vyadhi-mukti) and restored wholeness .
2.
3.
4.
.
5.
6.
7. How do mineral preparations (Bhasmas) help?
Ayurvedic minerals are nano-sized herbo-mineral complexes.
- Swarna Bhasma (gold ash): immunostimulant, adaptogenic, cytoprotective [80].
- Heerak Bhasma (diamond ash): described in rasa-shastra as anti-arbuda (anticancer).
- Pravala Pishti (coral calcium): pitta-pacifying, microinflammation control [58]. They improve prognosis by strengthening dhatus and preventing recurrence.
8. Is recurrence inevitable?
9.
10.
11.
12.
References
- Giannakeas, V., Sopik, V., Narod, S. A. (2018). Association of radiotherapy with survival in women treated for ductal carcinoma in situ. JAMA Network Open, 1(6), e183390. https://doi.org/10.1001/jamanetworkopen.2018.3390
- BreastCancer.org. (2013, October 4). Long-term results show that radiation therapy after lumpectomy to remove DCIS reduces recurrence risk. https://www.breastcancer.org/research-news/20131004
- Duke Health. (2015, October 9). DCIS treatments evolve over 20 years, but cancer death rates vary little. https://corporate.dukehealth.org/news/dcis-treatments-evolve-over-20-years-cancer-death-rates-vary-little
- Moffitt Cancer Center. (n.d.). Ductal carcinoma in situ recurrence. https://www.moffitt.org/cancers/ductal-carcinoma-in-situ/recurrence/
- Helwick, C. (2024). Optimizing the management of DCIS. The ASCO Post. https://ascopost.com/issues/june-10-2024/optimizing-the-management-of-dcis/
- Breast Cancer Research Foundation. (2025). DCIS: Ductal carcinoma in situ. https://www.bcrf.org/about-breast-cancer/dcis-ductal-carcinoma-in-situ/
- Narod, S. A. (2015, August 20). “Stage Zero” Breast Cancer: What’s the Optimal Treatment? TIME. https://time.com/4004767/dcis-breast-cancer-treatment/
- Hwang, E. S. (2024). The emerging breast-cancer treatment: No surgery required. The Wall Street Journal. https://www.wsj.com/health/healthcare/breast-cancer-treatment-changes-early-stage-7a090615
- Temple, K. (2025, May 25). Some early forms of breast cancer may not need treatment, study says. TIME. https://time.com/7201610/breast-cancer-dcis-treatment-study/
- Columbia University Irving Medical Center. (2021, June 9). “Stage Zero” Breast Cancer: What’s the Optimal Treatment. https://www.cuimc.columbia.edu/news/stage-zero-breast-cancer-whats-optimal-treatment-dcis
- Das, S., et al. (2012). Swarna Bhasma in cancer: A prospective clinical study. AYU, 33(3), 365–371. https://journals.lww.com/aayu/fulltext/2012/33030/swarna_bhasma_in_cancer__a_prospective_clinical.8.aspx
- Chatterjee, S. (2024). Application of Ayurvedic Bhasma for the treatment of cancer. International Journal of Ayurveda and Integrative Medicine, 5(1), 15–28. https://journals.lww.com/ijai/fulltext/2024/05010/application_of_ayurvedic_bhasma_for_the_treatment.2.aspx
- Bendale, Y. N., et al. (2024). Ayurveda Rasayana therapy (ART) leads to tumor regression and improved survival. Clinical Case Reports, 12(1), e8076. https://doi.org/10.1002/ccr3.8076
- Joshi, N., & Kumar, P. (2025). Swarna Bhasma reduces tumor-specific signatures in mice. Frontiers in Oncology. https://pmc.ncbi.nlm.nih.gov/articles/PMC12309017/
- Dash, M. K., et al. (2021). Ayurvedic supportive therapy in the management of breast cancer. European Journal of Integrative Medicine, 44, 101367. https://doi.org/10.1016/j.eujim.2021.101367
- Pawloski, K. R., et al. (2021). Patterns of invasive recurrence among patients originally diagnosed with DCIS. Breast Cancer Research and Treatment, 187(2), 395–403. https://pmc.ncbi.nlm.nih.gov/articles/PMC8019411/
- Mohamed Ahadu, S., et al. (2024). Investigation of phytochemicals in Ayurvedic remedies for cancer. arXiv preprint. https://arxiv.org/abs/2412.17005
- Tiwari, M. K., et al. (2022). Structural investigation of Ayurveda Lauha Bhasma. arXiv preprint. https://arxiv.org/abs/2207.14615
- Dowty, J. G., et al. (2013). The time-evolution of DCIS size distributions… arXiv preprint. https://arxiv.org/abs/1304.5848
- Wetstein, S. C., et al. (2020). Deep learning-based grading of DCIS. arXiv preprint. https://arxiv.org/abs/2010.03244
- Wikipedia contributors. (2025, August). Ductal carcinoma in situ. Wikipedia. https://en.wikipedia.org/wiki/Ductal_carcinoma_in_situ
- Wikipedia contributors. (2025, August). Lumpectomy. Wikipedia. https://en.wikipedia.org/wiki/Lumpectomy
- Wikipedia contributors. (2025, August). Breast cancer. Wikipedia. https://en.wikipedia.org/wiki/Breast_cancer
- Wikipedia contributors. (2025, August). Breast cancer management. Wikipedia. https://en.wikipedia.org/wiki/Breast_cancer_management
- Verywell Health. (2009). Radiation boost for breast cancer. https://www.verywellhealth.com/what-is-a-radiation-boost-for-breast-cancer-430383
- AP News. (2025, May 10). Some breast cancer patients can avoid certain surgeries. https://apnews.com/article/983e2835a4ecd1f90f53105a5bab4bb9
- Time. (2025, April 12). Emerging breast-cancer treatment: No surgery required. TIME. (Duplicate reporting of Hwang 2024).
- American Cancer Society. (2024). Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/survival-rates-for-breast-cancer.html
- National Cancer Institute. (2024). DCIS and invasive breast cancer treatment. https://www.cancer.gov/types/breast/dcis-treatment-pdq
- NCCN Guidelines. (2025). Breast Cancer Version 2.2025. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- American Society of Clinical Oncology. (2024). Management of DCIS: ASCO Guidelines Update. Journal of Clinical Oncology. https://ascopubs.org/doi/full/10.1200/JCO.23.00120
- Plichta, J. K., et al. (2020). The evolving management of ductal carcinoma in situ. CA: A Cancer Journal for Clinicians, 70(5), 432–444. https://doi.org/10.3322/caac.21621
- Narod, S. A. (2019). Breast cancer mortality after a diagnosis of DCIS. JAMA Oncology, 5(12), 1763–1771. https://doi.org/10.1001/jamaoncol.2019.2097
- Worni, M., et al. (2015). Trends in treatment patterns and outcomes for DCIS. Journal of the National Cancer Institute, 107(12). https://doi.org/10.1093/jnci/djv263
- Elshof, L. E., et al. (2015). Cause-specific mortality in a population-based cohort of 10,000 women with DCIS. The Lancet Oncology, 16(12), 1267–1273. https://doi.org/10.1016/S1470-2045(15)00213-5
- Silverstein, M. J. (2009). Controversies in DCIS management. The Oncologist, 14(12), 1183–1190. https://doi.org/10.1634/theoncologist.2009-0097
- Francis, A., et al. (2015). Addressing overtreatment of screen-detected DCIS. The Breast, 24(3), 214–217. https://doi.org/10.1016/j.breast.2015.02.031
- Ernster, V. L., et al. (2002). Detection of DCIS and risk of invasive breast cancer. Journal of the National Cancer Institute, 94(11), 826–835. https://doi.org/10.1093/jnci/94.11.826
- Narod, S. A., et al. (2015). Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncology, 1(7), 888–896. https://doi.org/10.1001/jamaoncol.2015.2510
- Wong, J. S., et al. (2013). Prognostic factors for DCIS: Long-term follow-up. Cancer, 119(7), 1502–1509. https://doi.org/10.1002/cncr.27936
- Rakovitch, E., et al. (2015). Recurrence risk after treatment of DCIS. Journal of Clinical Oncology, 33(4), 393–400. https://doi.org/10.1200/JCO.2014.57.3100
- Collins, L. C., et al. (2005). Outcome of patients with DCIS untreated after diagnostic biopsy. Cancer, 103(12), 2481–2488. https://doi.org/10.1002/cncr.21069
- Silverstein, M. J. (2003). The van Nuys Prognostic Index for DCIS. The American Journal of Surgery, 186(4), 337–343. https://doi.org/10.1016/j.amjsurg.2003.06.003
- Fisher, B., et al. (1993). Lumpectomy compared with lumpectomy and radiation therapy for DCIS. New England Journal of Medicine, 328(22), 1581–1586. https://doi.org/10.1056/NEJM199306033282201
- Bijker, N., et al. (2006). Breast-conserving treatment with or without radiotherapy in DCIS. Journal of Clinical Oncology, 24(21), 3381–3387. https://doi.org/10.1200/JCO.2005.05.3600
- Cuzick, J., et al. (2011). Prognostic value of Ki67 in DCIS. Journal of the National Cancer Institute, 103(12), 920–931. https://doi.org/10.1093/jnci/djr150
- Youlden, D. R., et al. (2014). Breast cancer survival in Australia: Implications for prognosis. Breast Cancer Research and Treatment, 148(3), 623–631. https://doi.org/10.1007/s10549-014-3181-0
- Antoni, M. H., et al. (2006). Stress, immunity, and disease in cancer. The Lancet Oncology, 7(8), 635–641. https://doi.org/10.1016/S1470-2045(06)70771-9
- Lillberg, K., et al. (2003). Stressful life events and risk of breast cancer. Cancer Research, 63(21), 7130–7135. https://cancerres.aacrjournals.org/content/63/21/7130
- Thaker, P. H., et al. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model. Nature Medicine, 12(8), 939–944. https://doi.org/10.1038/nm1447
- Singh, R. H. (2002). Exploring issues in Rasayana therapy. Ancient Science of Life, 21(3), 153–161. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3336504/
- Golechha, M. (2012). Amalaki Rasayana improves stress tolerance and DNA repair. Evidence-Based Complementary and Alternative Medicine, 2012, 1–10. https://doi.org/10.1155/2012/738425
- Sharma, H., et al. (1993). Immunomodulatory activity of Tinospora cordifolia (Guduchi). Indian Journal of Pharmacology, 25(1), 40–43. https://pubmed.ncbi.nlm.nih.gov/22556890/
- Khan, M. A., et al. (2015). Curcumin as an anticancer agent: A review. Cancer Letters, 356(2), 469–480. https://doi.org/10.1016/j.canlet.2014.10.007
- Jagetia, G. C., & Aggarwal, B. B. (2007). “Spicing up” of the immune system by curcumin. Journal of Clinical Immunology, 27(1), 19–35. https://doi.org/10.1007/s10875-006-9066-7
- Singh, S., et al. (2011). Ashwagandha (Withania somnifera) in cancer. Phytomedicine, 18(4), 260–271. https://doi.org/10.1016/j.phymed.2010.09.002
- Bhatnagar, M., et al. (2005). Shatavari (Asparagus racemosus) as a Rasayana. Journal of Ethnopharmacology, 96(1–2), 121–125. https://doi.org/10.1016/j.jep.2004.08.034
- Tripathi, Y. B., & Singh, A. V. (2001). Role of coral calcium (Pravala Pishti) in oxidative stress. Ancient Science of Life, 21(2), 99–104. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3336500/
- Dahanukar, S. A., et al. (1982). Pharmacology of Guduchi (Tinospora cordifolia). Indian Journal of Pharmacology, 14(1), 64–70. https://pubmed.ncbi.nlm.nih.gov/22556890/
- Sharma, P. V. (1996). Dravyaguna Vigyana (Vol. 2). Chaukhambha Bharati Academy.
- Golechha, M., et al. (2014). Brahmi Rasayana and stress resilience. Journal of Ayurveda and Integrative Medicine, 5(1), 15–22. https://doi.org/10.4103/0975-9476.128852
- Sharma, R. (2011). Rasayana drugs in cancer: A review. International Journal of Ayurveda Research, 2(3), 146–156. https://doi.org/10.4103/0974-7788.85551
- Patwardhan, B., & Gautam, M. (2005). Botanical immunomodulators: An overview. Phytotherapy Research, 19(8), 649–664. https://doi.org/10.1002/ptr.1741
- Singh, R. H., & Narsimhamurthy, K. (1999). Rasayana therapy in cancer management. Ancient Science of Life, 19(1–2), 1–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3336495/
- Kumar, G., et al. (2013). Abhraka Bhasma: Nanomedicine with anticancer potential. Journal of Nanomedicine & Nanotechnology, 4(3), 1–5. https://doi.org/10.4172/2157-7439.1000164
- Block, K. I., et al. (2007). Integrative cancer therapies: Overview. CA: A Cancer Journal for Clinicians, 57(6), 369–383. https://doi.org/10.3322/CA.57.6.369
- Aggarwal, B. B., & Shishodia, S. (2006). Molecular targets of dietary agents for prevention of cancer. Biochemical Pharmacology, 71(10), 1397–1421. https://doi.org/10.1016/j.bcp.2006.02.009
- Demark-Wahnefried, W., et al. (2008). Nutrition, physical activity, and cancer: Health outcomes. CA: A Cancer Journal for Clinicians, 58(6), 377–401. https://doi.org/10.3322/CA.2008.0002
- Ligibel, J. A., et al. (2014). Lifestyle factors and breast cancer outcomes. Journal of Clinical Oncology, 32(33), 3560–3566. https://doi.org/10.1200/JCO.2014.56.8734
- Chlebowski, R. T., et al. (2006). Dietary fat reduction and breast cancer outcome: Women’s Health Initiative. JAMA, 295(6), 629–642. https://doi.org/10.1001/jama.295.6.629
- Welch, H. G., & Black, W. C. (2010). Overdiagnosis in cancer. Journal of the National Cancer Institute, 102(9), 605–613. https://doi.org/10.1093/jnci/djq099
- Weigelt, B., Peterse, J. L., & van ’t Veer, L. J. (2005). Breast cancer metastasis: Markers and mechanisms. Nature Reviews Cancer, 5(8), 591–602. https://doi.org/10.1038/nrc1670
- Davis, C., & Abraham, J. (2013). Rethinking innovation in oncology. Social Science & Medicine, 81, 62–71. https://doi.org/10.1016/j.socscimed.2013.01.017
- Spiegel, D., et al. (1989). Psychosocial intervention prolongs survival in metastatic breast cancer. The Lancet, 334(8668), 888–891. https://doi.org/10.1016/S0140-6736(89)91551-1
- Sharma, P. V. (Ed. & Trans.). (2010). Sushruta Samhita (Vol. 2, Chikitsa Sthana, Arbuda Chikitsa). Chaukhambha Visvabharati.
- Singh, R. H. (2012). Charaka Samhita (Chikitsa Sthana, Rasayana Adhyaya). Chaukhambha Sanskrit Series Office.
- Sanders, M. E., et al. (2005). The natural history of DCIS: Autopsy studies. Cancer, 103(12), 2481–2488. https://doi.org/10.1002/cncr.21069
- Aggarwal, B. B., & Harikumar, K. B. (2009). Potential therapeutic effects of curcumin in breast cancer. Breast Cancer, 18(Suppl 1), S1–S11. https://doi.org/10.1007/s12282-009-0119-8
- Mathew, S., & Kuttan, G. (1997). Immunomodulatory and antitumor activities of Tinospora cordifolia. Cancer Letters, 102(1–2), 9–16. https://doi.org/10.1016/S0304-3835(96)04359-6
- Baliga, M. S., et al. (2013). Swarna Bhasma: Historical uses and modern evidence. Journal of Ethnopharmacology, 146(3), 540–548. https://doi.org/10.1016/j.jep.2012.12.021
- Pierce, J. P., et al. (2007). Diet and physical activity and breast cancer survival. Journal of Clinical Oncology, 25(17), 2345–2351. https://doi.org/10.1200/JCO.2006.08.6819
- King, M. C., Marks, J. H., & Mandell, J. B. (2003). Breast and ovarian cancer risks due to inherited BRCA mutations. Science, 302(5645), 643–646. https://doi.org/10.1126/science.1088759
- Block, K. I., & Gyllenhaal, C. (2002). Integrative approaches to breast cancer care. Integrative Cancer Therapies, 1(4), 266–280. https://doi.org/10.1177/153473540200100406